Abstract
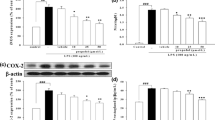
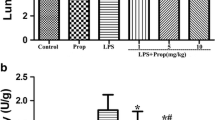
Nitric oxide is an active oxidant that contributes to the physiology and pathophysiology of macrophages. Propofol has been widely used in intravenous anesthesia. It possess antioxidant and immunomodulating effects. This study aimed to evaluate the effects of propofol on nitric oxide production in lipopolysaccharide-activated macrophages. Exposure of macrophages to propofol (25, 50 and 75 µM), to lipopolysaccharide (0.5, 1, 1.5 and 2 ng/ml) or to a combination of propofol and lipopolysaccharide did not affect cell viability. However, propofol at 100 µM led to significant cell death (P<0.05). The levels of nitrite, an oxidative product of nitric oxide, were increased in lipopolysaccharide-treated macrophages in a concentration-dependent manner (P<0.01), while propofol could concentration-dependently decrease the lipopolysaccharide-enhanced nitrite levels (P<0.01). Immunoblotting analysis revealed that lipopolysaccharide increased the protein level of inducible nitric oxide synthase (iNOS). The co-treatment of propofol and lipopolysaccharide significantly reduced this lipopolysaccharide-induced iNOS protein (357±49×103 versus 92±6×103 arbitrary units, P<0.01). Analysis by reverse transcriptase-polymerase chain reaction showed that lipopolysaccharide induced mRNA of iNOS, but that the inductive effect was inhibited by propofol (95±7×102 versus 30±4×102 arbitrary units, P<0.01). This study has demonstrated that propofol, at therapeutic concentrations, could suppress nitric oxide biosynthesis by inhibiting iNOS expression in lipopolysaccharide-activated macrophages. The mechanism of suppression was at a pretranslational level.




Similar content being viewed by others
References
Albina JE, Cui S, Mateo RB, Reichner JS (1993) Nitric oxide-mediated apoptosis in murine peritoneal macrophages. J Immunol 150:5080–5085
Beutler B, Poltorak A (2001) Sepsis and evolution of the innate immune response. Crit Care Med 29:S2–S6
Chang H, Tsai SY, Chang Y, Chen TL, Chen RM (2002) Therapeutic concentrations of propofol protects mouse macrophages from nitric oxide-induced cell death and apoptosis. Can J Anaesth 49:477–480
Chen RM, Liu HC, Lin YL, Jean WC, Chen JS, Wang JH (2002) Nitric oxide induces osteoblast apoptosis through the de novo synthesis of Bax protein. J Orthop Res 20:295–302
Chiou WF, Chou CJ, Chen CF (2001) Camptothecin suppresses nitric oxide biosynthesis in RAW 264.7 macrophages. Life Sci 69:625–635
Cudic M, Ducrocq C (2000) Transformations of 2,6-diisopropylphenol by NO-derived nitrogen oxides, particularly peroxynitrite. Nitric Oxide 4:147–156
Demiryurek AT, Cinel I, Kahraman S, Tecder-Unal M, Gogus N, Aypar U, Kanzik I (1998) Propofol and intralipid interact with reactive oxygen species: a chemiluminesecence study. Br J Anaesth 80:649–654
Horibe M, Ogawa K, Sohn JT, Murray PA (2000) Propofol attenuates acetylcholine-induced pulmonary vasorelaxation: role of nitric oxide and endothelium-derived hyperpolarizing factors. Anesthesiology 93:447–455
Kirkland TN, Finley F, Leturcq D, Moriarty A, Lee JD, Ulevitch RJ, Tobias PS (1993) Analysis of lipopolysaccharide binding by CD14. J Biol Chem 268:24818–24824
Kokita N, Hara A (1996) Propofol attenuates hydrogen peroxide-induced mechanical and metabolic derangements in the isolated rat heart. Anesthesiology 84:117–127
Kotani N, Hashimoto H, Sessler DI, Kikuchi A, Suzuki A, Takahashi S, Muraoka M, Matsuki A (1998) Intraoperative modulation of alveolar macrophage function during isoflurane and propofol anesthesia. Anesthesiology 89:1125–1132
Le WD, Colom LV, Xie W, Smith RG, Alexianu M, Appel SH (1995) Cell death induced by β-amyloid 1–40 in MES 23.5 hybrid clone: the role of nitric oxide and NMDA-gated channel activation leading to apoptosis. Brain Res 686:49–60
Liu HC, Chen RM, Jiang WC, Lin YL (2001) Cytotoxic and antioxidant effects of the water extract of the traditional Chinese herb gusuibu (Drynaria fortunei) on rat osteoblasts. J Formos Med Assoc 100:383–388
Lynn WA, Cohen J (1995) Management of septic shock. J Infect 30:207–212
Mikawa K, Akamatsu H, Nishina K, Shiga M, Maekawa N, Obara H, Niwa Y (1998) Propofol inhibits human neutrophil functions. Anesth Analg 87:695–700
Moncada S, Palmer RM, Higgs EA (1991) Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol Rev 43:109–142
Murphy PG, Myers DS, Davies MJ, Webster NR, Jones JG (1992) The antioxidant potential of propofol (2,6-diisopropylphenol). Br J Anaesth 68:613–618
Nathan C (1992) Nitric oxide as a secretory product of mammalian cells. FASEB J 6:3051–3064
Petros AJ, Bogle RG, Pearson JD (1993) Propofol stimulates nitric oxide release from cultured porcine aortic endothelial cells. Br J Pharmacol 109:6–7
Raetz CR, Ulevitch RJ, Wright SD, Sibley CH, Ding A, Nathan CF (1991) Gram-negative endotoxin: an extraordinary lipid with profound effects on eukaryotic signal transduction. FASEB J 5:2652–2660
Schuster JM, Nelson PS (2000) Toll receptors: an expanding role in our understanding of human disease. J Leukoc Biol 67:767–773
Sebel PS, Lowdon JD (1989) Propofol: a new intravenous anesthetic. Anesthesiology 71:260–277
Shimaoka M, Iida T, Ohara A, Taenaka N, Mashimo T, Honda T, Yoshiya I (1996) Ketamine inhibits nitric oxide production in mouse-activated macrophage-like cells. Br J Anaesth 77:238–242
West MA, Li MH, Seatter SC, Bubrick MP (1994) Pre-exposure to hypoxia or septic stimuli differentially regulates endotoxin release of tumor necrosis factor, interleukin-6, interleukin-1, prostaglandin E2, nitric oxide, and superoxide by macrophages. J Trauma 37:82–89
Yamamoto S, Kawana S, Miyamoto A, Ohshika H, Namiki A (1999) Propofol-induced depression of cultured rat ventricular myocytes is related to the M2-acetylcholine receptor–NO–cGMP signaling pathway. Anesthesiology 91:1712–1719
Young C, Knudsen N, Andrew H, Reves JG (2000) Sedation in the intensive care unit. Crit Care Med 28:854–866
Acknowledgements
The authors express their gratitude to Ms Sheau-Lan Tzeng and Ms Wan-Ju Lee for their technical support and data collection of the experiment. This study is supported by grants TMU90-Y05-A123 from Taipei Medical University and NSC90-2314-B-038-045 from the National Science Council, Taiwan, ROC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, RM., Wu, GJ., Tai, YT. et al. Propofol reduces nitric oxide biosynthesis in lipopolysaccharide-activated macrophages by downregulating the expression of inducible nitric oxide synthase. Arch Toxicol 77, 418–423 (2003). https://doi.org/10.1007/s00204-003-0453-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-003-0453-z