Abstract
Introduction
The purpose of this study was to obtain different structure measures as the three-dimensional (3D)-based scaling index method (SIM) and standard two-dimensional (2D) bone histomorphometric parameters from high-resolution (HR) magnetic resonance (MR) images of the distal radius and to compare these parameters with bone mineral density (BMD) in their diagnostic performance to differentiate postmenopausal patients with and without vertebral fractures.
Methods
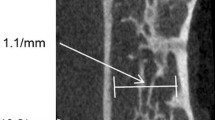
Axial HR-MR images of the distal radius were obtained at 1.5 T in 40 postmenopausal women (17 with osteoporotic spine fractures and 23 controls). Trabecular microarchitecture analysis was performed using the new structure measure \(m_{{P(\alpha )}}\), derived from the SIM, as well as standard morphological 2D parameters. BMD of the spine was obtained using quantitative computed tomography (QCT). Receiver operating characteristic (ROC) analyses were used to determine diagnostic performance in differentiating both groups. Results were validated by bootstrapping techniques.
Results
Significant differences between both patient groups were obtained using \(m_{{P(\alpha )}}\), 2D parameters, and spine BMD (p<0.05). In comparison with the 2D texture parameters [area under the curve (AUC) up to 0.67], diagnostic performance was significantly higher for \(m_{{P(\alpha )}}\)(AUC=0.85; p<0.05). There was a trend for a higher AUC value for \(m_{{P(\alpha )}}\)compared with BMD of the spine (AUC=0.71; p=0.81).
Conclusion
\(m_{{P(\alpha )}}\) yielded a robust measure of trabecular bone microarchitecture for HR-MR images of the radius, which significantly improved the diagnostic performance in differentiating postmenopausal women with and without osteoporotic spine fractures compared with standard 2D bone histomorphometric parameters. This 3D characterization of trabecular microarchitecture may provide a new approach to better assess the strength of human cancellous bone using HR-MR image data.






Similar content being viewed by others
References
Link TM, Majumdar S, Grampp S, Guglielmi G, van Kuijk C, Imhof H, Glueer C, Adams JE (1999) Imaging of trabecular bone structure in osteoporosis. Eur Radiol 9(9):1781–1788
Majumdar S (1998) A review of MR imaging of trabecular bone micro-architecture: contribution to the prediction of biomechanical properties and fracture prevalence. Technol Health Care 6:321–327
Ulrich D, van Rietbergen B, Laib A, Ruegsegger P (1999) The ability of three-dimensional structural indices to reflect mechanical aspects of trabecular bone. Bone 25:55–60
Wigderowitz C, Paterson C, Dashti H, McGurty D, Rowley D (2000) Prediction of bone strength from cancellous structure of the distal radius: Can we improve on DXA? Osteoporos Int 11:840–846
Chung HW, Wehrli FW, Williams JL, Wehrli SL (1995) Three dimensional nuclear magnetic resonance micro-imaging of trabecular bone. J Bone Miner Res 10:1452–1461
Jara H, Wehrli F, Chung H, Ford J (1993) High-resolution variable flip angle 3D MR imaging of trabecular microstructure in vivo. Magn Reson Med 29:528–539
Laib A, Newitt D, Lu Y, Majumdar S (2002) New model-independent measures of trabecular bone structure applied to in vivo high-resolution MR images. Osteoporos Int 13(2):130–136
Link TM, Majumdar S, Augat P, Lin JC, Newitt D, Lu Y, Lane NE, Genant HK (1998) In vivo high resolution MRI of the calcaneus: differences in trabecular structure in osteoporosis patients. J Bone Miner Res 13(7):1175–1182
Link T, Vieth V, Matheis J, Newitt D, Lu Y, Rummeny E, Majumdar S (2002) Bone structure of the distal radius and the calcaneus versus BMD of the spine and proximal femur in the prediction of osteoporotic spine fractures. Eur Radiol 12:401–408
Majumdar S, Genant HK, Grampp S, Newitt DC, Truong VH, Lin JC, Mathur A (1997) Correlation of trabecular bone structure with age, bone mineral density, and osteoporotic status: in vivo studies in the distal radius using high resolution magnetic resonance imaging. J Bone Miner Res 12(1):111–118
Majumdar S, Link T, Augat P, Lin J, Newitt D, Lane N, Genant H (1999) Trabecular bone architecture in the distal radius using MR imaging in subjects with fractures of the proximal femur. Osteoporos Int 10:231–239
Raeth C, Monetti R, Mueller D, Boehm HF, Rummeny E, Link T (2003) Analysing and selecting measures for quantifying trabecular bone structures using surrogates. Proc of the SPIE: medical imaging: image processing 5032:1748–1756
Boehm HF, Raeth C, Monetti R, Mueller D, Newitt D, Majumdar S, Rummeny E, Morfill G, Link T (2003) Local 3D scaling properties for the analysis of trabecular bone extracted from high-resolution magnetic resonance imaging of human trabecular bone: comparison with bone mineral density in the prediction of biomechanical strength in vitro. Invest Radiol 38(5):269–280
Gilsanz V, Loro ML, Roe TF, Sayre J, Gilsanz R, Schulz EE (1990) Vertebral size in elderly women with osteoporosis. Mechanical implications and relationship to fractures. J Clin Invest 95(5):2332–2337
Gilsanz V, Boechat M, Roe T, Loro M, Sayre J, Goodman W (1994) Gender differences in vertebral body sizes in adults: biomechanical implications. Radiology 190:678–682
Davis J, Grove J, Wasnich R, Ross P (1999) Spatial relationships between prevalent and incident spine fractures. Bone 24:261–264
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148
Adams R, Bischof L (1994) Seeded region growing. IEEE Trans Pattern Anal 16:641–646
Serra J (1988) Image analysis and mathematical morphology. Academic Press, London
Raeth C, Bunk W, Huber M, Morfill G, Retzlaff J, Schuecker P (2002) Analysing large scale structure: I. Weighted scaling indices and constrained randomisation. Mon Not R Astron Soc 337(2):413–421
Jamitzky F, Stark W, Bunk W, Thalhammer S, Raeth C, Aschenbrenner T, Morfill G, Heckl W (2000) Scaling-index method as an image processing tool in scanning-probe microscopy. Ultramicroscopy 86:241–246
Pompl R, Bunk W, Horst A, Stolz W, Abmayr W, Brauner W, Glaessl A, Morfill G (2000) MELDOQ: ein system zur unterstützung der früherkennung des malignen melanoms durch digitale bildverarbeitung. Bildverarbeitung für die Medizin. Springer, Berlin Heidelberg New York, pp 234–238
Lin J, Amling M, Newitt D, Selby K, Delling G, Genant H, Majumdar S (1996) Heterogeneity of trabecular bone structure in the calcaneus using high resolution magnetic resonance imaging (MRI). Osteoporosis Int 8:16–24
Parfitt M, Drezner M, Glorieux F, Kanis J, Malluche H, Meunier P, Ott S, Recker R (1987) Bone Histomorphometry: Standardization of nomenclature, symbols and units. Report of the ASBMR histomorphometry nomenclature committee. J Bone Miner Res 2:595–610
Efron B, Tibshirani R (1993) An introduction to the bootstrap. Chapman & Hall, New York
Majumdar S, Newitt D, Kothari M, Link T, Augat P, Lin J, Lang T, Genant H (1997) Measuring 3D trabecular structure and anisotropy using magnetic resonance. Osteoporosis Int 7:272
Wehrli FW, Ford JC, Chung HW, Wehrli SL, Williams JL, Grimm MJ, Kugelmass SD, Jara H (1993) The value of simple morphometric techniques in the diagnosis of osteoporosis. Calcif Tissue Int 53(1):162–169
Majumdar S, Newitt D, Jergas M, Gies A, Chiu E, Osman D, Keltner J, Keyak J, Genant H (1995) Evaluation of technical factors affecting the quantification of trabecular bone structure using magnetic resonance imaging. Bone 17(4):417–430
Newitt DC, van Rietbergen B, Majumdar S (2002) Processing and analysis of in vivo high-resolution MR images of trabecular bone for longitudinal studies: reproducibility of structural measures and micro-finite element analysis derived mechanical properties. Osteoporos Int 13(4):278–287
Duboeuf F, Jergas M, Schott A, Wu C, Gluer C, Genant H (1995) A comparison of bone densitometry measurements of the central skeleton in post-menopausal women with and without vertebral fracture. Br J Radiol 68:747–753
Newitt D, Majumdar S, van Rietbergen B, von Ingersleben G, Harris S, Genant H, Chesnut C, Garnero P, MacDonald B (2002) In vivo assessment of architecture and micro-finite element analysis derived indices of mechanical properties of trabecular bone in the radius. Osteoporos Int 13(1):6–17
Boutry N, Cortet B, Dubois P, Marchandise X, Cotten A (2003) Trabecular bone structure of the calcaneus: preliminary in vivo MR imaging assessment in men with osteoporosis. Radiology 227(3):708–717
Wehrli FW, Gomberg BR, Saha PK, Song HK, Hwang SN, Snyder PJ (2001) Digital topological analysis of in vivo magnetic resonance microimages of trabecular bone reveals structural implications of osteoporosis. J Bone Miner Res 16(8):1520–1531
Acknowledgements
The authors thank T. Aschenbrenner, W. Bunk, F. Jamitzky, and R. Pompl for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the grant “Tandem-Projekt: Verbesserung der Diagnose von Osteoporose” of the Max-Planck-Society.
Appendix
Appendix
Calculation of weighted scaling indices
The 3D HR-MR image data are represented as a virtual 4D point distribution, where the fourth dimension is depicted by the grey level of each point. Thus, spatial and intensity information of each voxel i is integrated in a 4D vector \({\overrightarrow{p}} _{i} = (x_{i} ,y_{i} ,z_{i} ,g_{i} )\), where x, y, and z denote the spatial variables and g the intensity of the considered voxel. Given this representation of the 3D image data, we calculated for each point with coordinate vector \({\overrightarrow{p}} _{i} \) the weighted cumulative point distribution \(\rho (\overset{\lower0.5em\hbox{$\smash{\scriptscriptstyle{\rightharpoonup}}$}} {p} _{i} ,r)\) using a Gaussian shaping function:
d ij denotes the (Euclidean) distance between two points \({\overrightarrow{p}} _{i}\) and \({\overrightarrow{p}} _{j} .\)
This formula can be interpreted in the following way: For each point \({\overrightarrow{p}} _{i} \), one counts the number of adjacent points located in the vicinity of this point. Shaping, or equivalently, the kernel function introduces a weight, with which the point \({\overrightarrow{p}} _{j} \) contributes to the total sum. Thereby, it is ensured that points with a smaller distance to \({\overrightarrow{p}} _{i} \) have a higher weight than those with higher distances.
Local scaling properties, which can be regarded as a measure for the dimensionality of the point set, are, as usual, assessed by analyzing the change in the point distribution \(\rho {\left( {{\overrightarrow{p}} _{i} ,r} \right)}\) with varying scale parameter r. Therefore, the weighted scaling indices \(\alpha {\left( {{\overrightarrow{p}} _{i} ,r} \right)}\) for each point are given by the logarithmic derivative of \(\rho (\overset{\lower0.5em\hbox{$\smash{\scriptscriptstyle\rightharpoonup}$}} {p} _{i} ,r)\) with respect to\(\alpha {\left( {{\overrightarrow{p}} _{i} ,r} \right)} = \frac{{\partial \ln \rho {\left( {{\overrightarrow{p}} _{i} ,r} \right)}}}{{\partial \ln r}}\).
Using the differentiable Gaussian kernel function, we obtained an analytic expression for arbitrary values of r:
Since the quality of the measure strongly depends on the choice in relationship to the objects of the analysis—in our case: trabeculae—it is essential to have a certain amount of initial information about the nature of the investigated point distribution with respect to the scale of the structuring elements.
Using the 4D representation of image data and exploiting local scaling properties in this embedding space, the SIM offers the possibility of taking advantage of a fully 3D analysis of bone structure. Scaling indices for the whole point set under study form the probability distribution
.
This representation of point distribution can be regarded as structural decomposition of the point set where points are differentiated according to local morphological features of the structure elements to which they belong to. Thus, the spectrum reveals the structural content of a point set and can consequently characterize the image data under study.
Rights and permissions
About this article
Cite this article
Mueller, D., Link, T.M., Monetti, R. et al. The 3D-based scaling index algorithm: a new structure measure to analyze trabecular bone architecture in high-resolution MR images in vivo. Osteoporos Int 17, 1483–1493 (2006). https://doi.org/10.1007/s00198-006-0130-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-006-0130-1