Abstract
Because the diaphragm is essential for survival, we wondered if it might be less vulnerable to the long-lasting effects of fatigue than limb muscles. Using a recently introduced magnetic probe to activate the phrenic nerves, we followed the evolution of twitch transdiaphragmatic pressure after inducing fatigue in healthy volunteers. Twenty-four hours after its induction, diaphragmatic fatigue had not fully recovered. Findings from this study later served as the foundation for incorporating a once-daily, T-tube-trial arm into a randomized controlled trial of techniques for ventilator weaning in intensive care unit patients and also influenced the design of a controlled trial of the weaning of tracheostomy patients who required prolonged ventilation. The research methodology was later employed to determine whether low-frequency fatigue is responsible for weaning failure. Employing a further modification of the technique—twitch airway pressure—it became evident that respiratory muscle weakness is a greater problem than fatigue in ventilated patients. Twitch airway pressure is now being used to document the prevalence and consequences of ventilator-induced respiratory muscle weakness. Our study—which began with a circumscribed, simple question—has yielded dividends in unforeseen directions, illustrating the fruitfulness of research into basic physiological mechanisms.
Similar content being viewed by others
In 1995, we published a study in the Journal of Applied Physiology where we assessed the time course of recovery from diaphragmatic fatigue [1]. Significant fatigue—defined as effort-induced decrease in muscle contractility that recovers after rest—was still evident 24 h after its induction in healthy volunteers. We discuss the impact of this paper on understanding of respiratory muscle function in critically ill patients. We begin by reviewing the technical challenges for research on diaphragmatic fatigue in the early 1990s. After reviewing the key findings in our study, we discuss how the results influenced the design of randomized controlled trials of ventilator weaning and led to the introduction of a simple technique to screen for the occurrence of respiratory muscle atrophy in mechanically ventilated patients [2].
In the early 1980s, the standard approach for studying diaphragmatic fatigue was to construct a force–frequency curve (Fig. 1) [3, 4]. This method entailed activation of one phrenic nerve with an external probe, which delivered electrical shocks at frequencies between 10 and 100 Hz. This technique is extremely painful because a high voltage is needed to overcome the resistance of the skin. The inevitable tetanus-like contraction of neck muscles, particularly at high stimulation frequencies, is a further source of pain [4, 5]. The procedure demands considerable manual skill on the part of the investigator because simultaneous activation of the neck muscles causes displacement of the external electrode, making it difficult to maintain a maximal stimulus [4]. Another technical challenge is the difficulty in achieving careful control over chest-wall geometry because activation of one phrenic nerve (normally two fire at the same time) induces diaphragmatic distortion, which interferes with the generation of transdiaphragmatic pressure [6].
Constructing a force–frequency curve. Left panel following placement of an esophageal and a gastric balloon, a subject undergoes unilateral electrical stimulations of the phrenic nerve at various stimulation frequencies. Right panel force–frequency curve of the diaphragm recorded in three healthy men. The transdiaphragmatic pressure (Pdi) of each subject is expressed as a percentage of his maximal stimulated transdiaphragmatic pressure. Values are mean ± standard deviation. (Right panel used with permission from [4])
On the basis of the contour of force–frequency curves, two different types of muscle fatigue have been recognized. Low-frequency fatigue, signified by a decrease in the amount of force occurring at stimulation frequencies of 10–20 Hz, is thought to be caused by irreversible disruption of calcium release in the cytoplasm and muscle damage [7, 8]; it has a slow rate of recovery. High-frequency fatigue, signified by a decrease in force at stimulation frequencies of 50–100 Hz [9], results from various mechanisms related to intracellular concentrations of calcium and inorganic phosphate; it has a rapid rate of recovery [10]. During normal regular breathing, the peak frequency of motor neurons for the phrenic nerves is 7–14 Hz [11], and, thus, low-frequency fatigue is judged to have greater clinical importance than high-frequency fatigue [12–14].
The challenges in constructing force–frequency curves (for subjects and investigators) led to modifications of the technique in the mid-1980s [5, 15]. It was recognized that stimulation of the phrenic nerves at a frequency of 1 Hz produced changes in transdiaphragmatic pressure that paralleled the pressures induced by stimulation at 20 Hz [16]. That is, external activation of the phrenic nerves at 1 Hz—subsequently dubbed twitch stimulation [5, 15]—could be used to detect low-frequency fatigue of the diaphragm [16]. Although this approach represented a significant advance, it also had limitations. The use of a needle electrode for nerve activation sometimes produced inadvertent injuries [17]. When a transcutaneous electrode was employed, it was difficult or impossible to locate the phrenic nerves in over a quarter of patients with respiratory disease [18]. Maintaining a constant symmetrical maximal stimulus can be difficult, and so repetitive twitch stimulations are commonly performed [15]. This approach, unfortunately, produces artifactual increases in twitch pressure: so-called staircase potentiation (an increase in muscle contractility that occurs after a series of low-frequency stimulations) [19]. Consequently, pressure tracings are distorted and rendered uninterpretable. The high voltage (80–160 V) required to overcome skin resistance with use of a transcutaneous electrode caused considerable pain [20]. Because of these problems, phrenic nerve stimulation with electrical electrodes (needle or transcutaneous) remained a difficult technique to use—and would never have been feasible for use in the intensive care unit (ICU).
Aware of the technical limitations of electrical stimulation, industry developed an alternative approach: magnetic stimulation of the phrenic nerves [21]. This approach had a number of attractions. It was easier to guarantee neural activation because the magnetic probe generates a broad field of activity over the neck, in contrast with the need to precisely position an electric probe over the phrenic nerve (Fig. 2). Consequently, the likelihood of inducing staircase potentiation is lessened. Magnetic stimulation is also considerably less painful than electrical stimulation [21–25]. Importantly, the transdiaphragmatic pressure generated by magnetic stimulation corresponds to the pressure observed with electrical stimulation of the phrenic nerves at 1 Hz [22].
Magnetic stimulation of the phrenic nerves. Left panel posterior approach: a circular magnetic probe is centered over the spinous process of the seventh cervical vertebra. When activated, the probe generates a broad magnetic field that causes bilateral stimulation of the phrenic nerve roots. Right panel anterolateral approach: two figure-of-eight probes are positioned adjacent to the posterior border of the sternomastoid muscle at the level of the cricoid cartilage
Given these technical advances, we decided to employ magnetic stimulation to study the time course of recovery from diaphragmatic fatigue [1]. The study was motivated by our awareness of research on limb muscles that demonstrated evidence of fatigue 24 h after its artificial induction [26, 27]. In 1992, information on the pattern of recovery from diaphragmatic fatigue was limited to 1 h [28]. Because the diaphragm is essential for vital function there was every reason to suspect that it might display a faster recovery pattern than that observed in limb muscles. To characterize the timeline of recovery, we performed repeated stimulations of the phrenic nerve during the first hour after induction of fatigue, at 8 h, and again at 24 h. Such measurements demanded considerable stoicism on the part of our volunteers. Before the study got underway, we recognized that we needed to have meticulous control over post-tetanic potentiation (the transient increase in muscle contractility that occurs after a forceful contraction elicited by high-frequency volleys of nerve depolarization) [19].
For our study into recovery of diaphragmatic fatigue, we invited 12 healthy individuals (mostly fellow investigators and ourselves) to participate. We employed a standard approach to induce diaphragmatic fatigue: the subjects breathed through a small aperture, which was progressively narrowed so that they had to generate 60 % of maximal transdiaphragmatic pressure with every breath. An investigator stood at the side and ensured that the subject never slacked off and continued to generate the target pressure until he or she could go no further—a point that has been dubbed “task failure” and is taken as evidence of diaphragmatic fatigue. At baseline, twitch transdiaphragmatic pressure was 38.9 cmH2O, and it fell to 25.1 cmH2O 10 min after conclusion of the fatigue protocol (Fig. 3). Twitch pressure increased by only 2.5 cmH2O 1 h later and by 6.5 cmH2O after 8 h. The increase in twitch pressure over the ensuing 16 h was miniscule, 1.1 cmH2O; that is, 24 h after completion of the fatigue protocol, diaphragmatic twitch pressure was 16 % below baseline. Mechanisms that explain long-lasting diaphragmatic fatigue include irreversible disruption of calcium kinetics [7], activation of calpain (a calcium-dependent nonlysosomal protease), increased muscle temperature, excessive production of reactive oxygen species, and eccentric contractions (contraction of a muscle at a time while it is being simultaneously stretched by an external force) [8].
Induction of diaphragmatic fatigue (stippled bar) produced a significant fall in transdiaphragmatic twitch pressure (Pditw); the fatigue was induced by requesting 12 healthy volunteers to breathe through a resistor until they could go no further. The nadir in twitch pressure was reached at 10 min after completing the fatigue protocol, and the values at 8 and 24 h were significantly below the baseline value. Values are mean ± standard error. *Significant difference compared with baseline value, p < 0.01. (With permission from [1])
Only one research group has subsequently undertaken a study broadly similar to ours [29]. Like us, Travaline et al. [29] found that twitch transdiaphragmatic pressure was significantly depressed at 30 min and 1 h after fatigue was induced in healthy volunteers. At 25 h, recovery was complete, whereas we had observed that twitch pressures were significantly below baseline at 24 h. The discrepancy can be explained by the more extreme load employed by Travaline et al. [29]: their subjects had to generate 80 % of maximal transdiaphragmatic pressure, whereas ours generated 60 %. Very large respiratory loads activate reflex mechanisms that inhibit central neural output (a phenomenon that has been called central fatigue) [30]. This decrease in neural output reduces activation of the diaphragm during extreme loading [31], and protects the muscle against contractile fatigue and muscle injury [30].
As we were planning and conducting our study on the pattern of recovery from diaphragmatic fatigue, one of us (MJT) was designing a randomized controlled trial (RCT) of weaning techniques, which was carried out in Spain [32]. Because our preliminary data suggested that the diaphragm was every bit as slow as limb muscles in recovering from fatigue, MJT decided to include a study arm of a once-daily T-tube trial in the RCT, an approach that was distinctly unusual at that time (the usual approach being to employ multiple T-tube trials several times a day) [33]. When the RCT was completed, the rate of successful weaning was almost threefold higher with a once-daily T-tube trial than with intermittent mandatory ventilation and twofold higher than with pressure support [32]. The findings of this RCT blended convincingly with the results of our study on the pattern of recovery from diaphragmatic fatigue in healthy volunteers. Given the stress imposed on the respiratory muscles in a patient who fails a T-tube trial, and the fact that it takes at least 24 h to recover from such stress, it makes little sense to undertake T-tube trials more often than once a day. The stress imposed on the respiratory muscles during a once-daily T-tube trial, combined with the period of rest over the subsequent 23 h or so, is ideally suited to eliciting adaptive changes in the respiratory muscles [34].
An RCT operates on the basis of probabilism, and one may obtain a result without knowing whether or not it necessarily makes sense [35]. Contrasted with statistical probabilism, the aim of science is to gain knowledge of how nature works [36]; an understanding of physiological mechanism is the basis for generalization and sound clinical practice [37]. About 90 % of RCTs in critically ill patients have “negative results.” One explanation for this sorry state of affairs is that many RCTs are not well grounded on physiological principles. The study on recovery from diaphragmatic fatigue not only served as the motivation for incorporating a once-daily T-tube arm in an RCT of ventilator weaning in ICU patients, but the findings also influenced the design of the recent RCT in patients receiving prolonged ventilation, which demonstrated a rate of successful weaning 1.43 times faster with trials of unassisted breathing (through a tracheostomy collar) than with pressure support [38].
Having demonstrated that twitch transdiaphragmatic pressure was sufficiently robust to elucidate the time course of recovery from diaphragmatic fatigue—and, most importantly, that the technique was acceptable to editors and reviewers as a reliable method for documenting the presence of low-frequency fatigue—we were emboldened to tackle the $64,000 question: Do patients who fail a trial of weaning from mechanical ventilation develop low-frequency diaphragmatic fatigue?
In the early 1990s, it was widely assumed that the dominant reason that patients failed trials of ventilator weaning was because of diaphragmatic fatigue [39–45]. Yet, the arguments supporting this possibility largely consisted of armchair reasoning and speculation. Investigators in the field recognized that non-voluntary measurements of diaphragmatic contractility were needed but this was an enormous challenge [46]. It was difficult enough to perform phrenic nerve stimulation, and control for confounding factors such as end-expiratory lung volume and twitch potentiation in healthy volunteers; it was far from clear that it would be possible to obtain high fidelity, reliable recordings in critically ill patients as they failed a weaning trial.
With growing expertise with magnetic stimulation, we decided to find out whether patients who fail a trial of ventilator weaning develop low-frequency fatigue of the diaphragm [47]. We recruited 19 patients, 11 of whom went on to fail a T-tube trial and eight of whom passed the trial. Before the trial, twitch transdiaphragmatic pressure was 10.3 cmH2O in the weaning-success patients and 8.9 cmH2O in the weaning-failure patients. Thirty minutes after completion of the T-tube trial, twitch pressure was not different from the baseline value in either the success patients (11.2 cmH2O) or the failure patients (9.4 cmH2O). On the basis of these results, some authors have concluded that weaning-failure patients are not at risk of developing diaphragmatic fatigue. This interpretation is erroneous, as it misses an important nuance in our study. The T-tube trials were terminated by clinicians at a point where a patient exhibited physical signs of significant distress; we cannot say whether fatigue would, or would not, have occurred had the trials been conducted a few minutes longer [8]. That is, the “negative findings” in this study do not justify a laissez-faire approach to weaning—the willy-nilly undertaking of a T-tube trial in any ventilated patient.
At the time we were conducting studies of fatigue during weaning, we also wondered if it might be possible to simplify the methodology and use airway pressure as a surrogate for transdiaphragmatic pressure, and thus avoid the need for inserting esophageal and gastric balloons [48, 49]. (The London group of John Moxham was also studying the same issue [50].) In healthy volunteers, we found close concordance between twitch airway pressures and twitch transdiaphragmatic pressures. Satisfactory tracings were obtained only if the subject was capable of performing a prespecified maneuver that prevented closure of the glottis during magnetic stimulation.
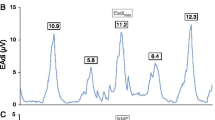
Glottic closure cannot arise in a patient with an endotracheal tube. In ventilated patients, we found a good correlation between twitch airway pressures and twitch transdiaphragmatic pressures (Fig. 4) [51]. The limits of agreement were wide, meaning that a particular twitch airway pressure was a poor predictor of twitch transdiaphragmatic pressure. (The London group reported similar results [52].) Yet, the twitch airway pressures were extremely reproducible, meaning that they could be used to track changes in diaphragmatic contractility in ventilated patients [51].
Airway pressures and electromyographic (EMG) signals of the right and left hemidiaphragm in an intubated patient. Following phrenic nerve stimulation, muscle depolarization produces compound motor action potentials which are followed by the equivalent falls in airway pressure (broken line) and esophageal pressure (continuous line)
The twitch airway pressures that we [51] and the London group [52] recorded in intubated patients (−8.2 and −6.7 cmH2O, respectively) were only a fraction of the values observed in healthy volunteers (−16 cmH2O) [53]. Impaired muscle contractility can result from fatigue or weakness. Fatigue is an implausible mechanism for the low twitch pressures in these patients because the patients had received mechanical ventilation for several days before the measurements [54]. Instead, the low twitch airway pressures signify muscle weakness, raising the possibility of ventilator-induced respiratory muscle injury and atrophy [55–57]. This possibility is supported by the recent data of Jaber et al. [58], who reported the early development of profound weakness and injury in ventilated patients. Demoule et al. [59] have also used twitch airway pressure to document the prevalence and risk factors for ventilator-associated respiratory muscle weakness and its impact on clinical outcomes.
Our study [1] began with a simple, confined question: Is the diaphragm immune from the long-lasting effects of fatigue observed in limb muscles? The findings have acted as both tinder and match, igniting fires in unexpected locations, including the introduction of an innovative approach to weaning (use of once-daily T-tube trials) and the implementation of a non-volitional test of respiratory muscle strength, as opposed to fatigue (making it possible to diagnose and follow the course of ventilator-induced respiratory muscle weakness). The study serves as an example of the fruitfulness and expected dividends that result from research conducted into basic physiological mechanisms.
References
Laghi F, D’Alfonso N, Tobin MJ (1995) Pattern of recovery from diaphragmatic fatigue over 24 h. J Appl Physiol 79:539–546
Anzueto A, Tobin MJ, Moore G (1987) Effect of prolonged mechanical ventilation on diaphragmatic function: a preliminary study of a baboon model. Am Rev Respir Dis 135:A201
Aubier M, De Troyer A, Sampson M, Macklem PT, Roussos C (1981) Aminophylline improves diaphragmatic contractility. N Engl J Med 305:249–252
Moxham J, Morris AJ, Spiro SG, Edwards RH, Green M (1981) Contractile properties and fatigue of the diaphragm in man. Thorax 36:164–168
Aubier M, Murciano D, Lecocguic Y, Viires N, Pariente R (1985) Bilateral phrenic stimulation: a simple technique to assess diaphragmatic fatigue in humans. J Appl Physiol 58:58–64
Bellemare F, Bigland-Ritchie B, Woods JJ (1986) Contractile properties of the human diaphragm in vivo. J Appl Physiol 61:1153–1161
Verburg E, Murphy RM, Richard L, Lamb GD (2009) Involvement of calpains in Ca2+-induced disruption of excitation-contraction coupling in mammalian skeletal muscle fibers. Am J Physiol Cell Physiol 296:C1115–C1122
Laghi F, Tobin MJ (2003) Disorders of the respiratory muscles. Am J Respir Crit Care Med 168:10–48
Aubier M, Farkas G, De Troyer A, Mozes R, Roussos C (1981) Detection of diaphragmatic fatigue in man by phrenic stimulation. J Appl Physiol 50:538–544
Allen DG, Lamb GD, Westerblad H (2008) Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88:287–332
De Troyer A, Leeper JB, McKenzie DK, Gandevia SC (1997) Neural drive to the diaphragm in patients with severe COPD. Am J Respir Crit Care Med 155:1335–1340
Mador MJ, Acevedo FA (1985) Effect of respiratory muscle fatigue on subsequent exercise performance. J Appl Physiol 70:2059–2065
Yan S, Lichros I, Zakynthinos S, Macklem PT (1993) Effect of diaphragmatic fatigue on control of respiratory muscles and ventilation during CO2 rebreathing. J Appl Physiol 75:1364–1370
Laghi F, Topeli A, Tobin MJ (1998) Does resistive loading decrease diaphragmatic contractility before task failure? J Appl Physiol 85:1103–1112
Bellemare F, Bigland-Ritchie B (1984) Assessment of human diaphragm strength and activation using phrenic nerve stimulation. Respir Physiol 58:263–277
Ferguson GT (1994) Use of twitch pressures to assess diaphragmatic function and central drive. J Appl Physiol 77:1705–1715
Al-Shekhlee A, Shapiro BE, Preston DC (2003) Iatrogenic complications and risks of nerve conduction studies and needle electromyography. Muscle Nerve 27:517–526
Mills G, Kyroussis D, Hamnegard C, Wragg S, Green M, Moxham J (1994) Evaluation of hemidiaphragmatic contractility by unilateral magnetic phrenic nerve stimulation. Eur Respir J 8:339S (abstract)
Vandenboom R, Gittings W, Smith IC, Grange RW, Stull JT (2013) Myosin phosphorylation and force potentiation in skeletal muscle: evidence from animal models. J Muscle Res Cell Motil 34:317–332
Mier A, Brophy C, Moxham J, Green M (1989) Twitch pressures in the assessment of diaphragm weakness. Thorax 44:990–996
Similowski T, Fleury B, Launois S, Cathala HP, Bouche P, Derenne JP (1989) Cervical magnetic stimulation: a new painless method for bilateral phrenic nerve stimulation in conscious humans. J Appl Physiol 67:1311–1318
Laghi F, Harrison MJ, Tobin MJ (1996) Comparison of magnetic and electrical phrenic nerve stimulation in assessment of diaphragmatic contractility. J Appl Physiol 80:1731–1742
Man WD, Moxham J, Polkey MI (2004) Magnetic stimulation for the measurement of respiratory and skeletal muscle function. Eur Respir J 24:846–860
Duguet A, Demoule A, Gonzalez J, Remy-Neris O, Derenne JP, Similowski T (2006) Predicting the recovery of ventilatory activity in central respiratory paralysis. Neurology 67:288–292
Hopkinson NS, Sharshar T, Dayer MJ, Lofaso F, Moxham J, Polkey MI (2012) The effect of acute non-invasive ventilation on corticospinal pathways to the respiratory muscles in chronic obstructive pulmonary disease. Respir Physiol Neurobiol 183:41–47
Edwards RH, Hill DK, Jones DA, Merton PA (1977) Fatigue of long duration in human skeletal muscle after exercise. J Physiol 272:769–778
Newham DJ, Mills KR, Quigley BM, Edwards RH (1983) Pain and fatigue after concentric and eccentric muscle contractions. Clin Sci (Lond) 64:55–62
Yan S, Similowski T, Gauthier AP, Macklem PT, Bellemare F (1992) Effect of fatigue on diaphragmatic function at different lung volumes. J Appl Physiol 72:1064–1067
Travaline JM, Sudarshan S, Criner GJ (1997) Recovery of PdiTwitch following the induction of diaphragm fatigue in normal subjects. Am J Respir Crit Care Med 156:1562–1566
Gandevia SC (2001) Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81:1725–1789
Bellemare F, Bigland-Ritchie B (1987) Central components of diaphragmatic fatigue assessed by phrenic nerve stimulation. J Appl Physiol 62:1307–1316
Esteban A, Frutos F, Tobin MJ, Alia I, Solsona JF, Valverdu I, Fernandez R, de la Cal MA, Benito S, Tomas R, Spanish Lung Failure Collaborative Group (1995) A comparison of four methods of weaning patients from mechanical ventilation. N Engl J Med 332:345–350
Tobin MJ, Jubran A (2013) Weaning from mechanical ventilation. In: Tobin MJ (ed) Principles and practice of mechanical ventilation, 3rd edn. McGraw Hill, New York, pp 1293–1306
Faulkner JA (1995) Structural and functional adaptations of skeletal muscle. In: Roussos C (ed) The thorax. Part A: physiology, 2nd edn. Marcel Dekker Inc, New York, pp 269–294
Rothman KJ (2002) Epidemiology: an introduction. Oxford University Press, New York, pp 20–21
Reichenbach H (1951) The rise of scientific philosophy. University of California Press, Berkeley, pp 5–7
Tobin MJ, Jubran A (2008) Meta-analysis under the spotlight: focused on a meta-analysis of ventilator weaning. Crit Care Med 36:1–7
Jubran A, Grant BJ, Duffner LA, Collins EG, Lanuza DM, Hoffman LA, Tobin MJ (2013) Effect of pressure support vs unassisted breathing through a tracheostomy collar on weaning duration in patients requiring prolonged mechanical ventilation: a randomized trial. JAMA 309:671–677
Cohen CA, Zagelbaum G, Gross D, Roussos C, Macklem PT (1982) Clinical manifestations of inspiratory muscle fatigue. Am J Med 73:308–316
Brochard L, Harf A, Lorino H, Lemaire F (1989) Inspiratory pressure support prevents diaphragmatic fatigue during weaning from mechanical ventilation. Am Rev Respir Dis 139:513–521
Goldstone JC, Green M, Moxham J (1994) Maximum relaxation rate of the diaphragm during weaning from mechanical ventilation. Thorax 49:54–60
Tobin MJ, Perez W, Guenther SM, Lodato RF, Dantzker DR (1987) Does rib cage-abdominal paradox signify respiratory muscle fatigue? J Appl Physiol 63:851–860
Roussos C, Macklem PT (1982) The respiratory muscles. N Engl J Med 307:786–797
Grassino A, Macklem PT (1984) Respiratory muscle fatigue and ventilatory failure. Annu Rev Med 35:625–647
Rochester DF (1993) Respiratory muscles and ventilatory failure: 1993 perspective. Am J Med Sci 305:394–402
Tobin MJ, Laghi F (1998) Monitoring respiratory muscle function. In: Tobin MJ (ed) Principles and practice of intensive care monitoring. McGraw-Hill, New York, pp 497–544
Laghi F, Cattapan SE, Jubran A, Parthasarathy S, Warshawsky P, Choi YS, Tobin MJ (2003) Is weaning failure caused by low-frequency fatigue of the diaphragm? Am J Respir Crit Care Med 167:120–127
Laghi F, Tobin MJ (1997) Relationship between transdiaphragmatic and mouth twitch pressures at functional residual capacity. Eur Respir J 10:530–536
Topeli A, Laghi F, Tobin MJ (1999) Can diaphragmatic contractility be assessed by twitch airway pressures in patients with chronic obstructive pulmonary disease? Am J Respir Crit Care Med 160:1369–1374
Hamnegard CH, Wragg S, Kyroussis D, Mills G, Bake B, Green M, Moxham J (1995) Mouth pressure in response to magnetic stimulation of the phrenic nerves. Thorax 50:620–624
Cattapan SE, Laghi F, Tobin MJ (2003) Can diaphragmatic contractility be assessed by airway twitch pressure in mechanically ventilated patients? Thorax 58:58–62
Watson AC, Hughes PD, Louise HM, Hart N, Ware RJ, Wendon J, Green M, Moxham J (2001) Measurement of twitch transdiaphragmatic, esophageal, and endotracheal tube pressure with bilateral anterolateral magnetic phrenic nerve stimulation in patients in the intensive care unit. Crit Care Med 29:1325–1331
Kabitz HJ, Walterspacher S, Walker D, Windisch W (2007) Inspiratory muscle strength in chronic obstructive pulmonary disease depending on disease severity. Clin Sci (Lond) 113:243–249
Tobin MJ, Laghi F, Jubran A (2010) Narrative review: ventilator-induced respiratory muscle weakness. Ann Intern Med 153:240–245
Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, Powers SK, Shrager JB (2008) Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 358:1327–1335
Vassilakopoulos T (2013) Ventilator-induced diaphargmatic dysfunction. In: Tobin MJ (ed) Principles and practice of mechanical ventilation, 3rd edn. McGraw Hill, New York, pp 1025–1040
Supinski GS, Ann CL (2013) Diaphragm weakness in mechanically ventilated critically ill patients. Crit Care 17:R120
Jaber S, Petrof BJ, Jung B, Chanques G, Berthet JP, Rabuel C, Bouyabrine H, Courouble P, Koechlin-Ramonatxo C, Sebbane M, Similowski T, Scheuermann V, Mebazaa A, Capdevila X, Mornet D, Mercier J, Lacampagne A, Philips A, Matecki S (2011) Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med 183:364–371
Demoule A, Jung B, Prodanovic H, Molinari N, Chanques G, Coirault C, Matecki S, Duguet A, Similowski T, Jaber B (2013) Diaphragm dysfunction on admission to the intensive care unit. Prevalence, risk factors, and prognostic impact-a prospective study. Am J Respir Crit Care Med 188:213–219
Acknowledgments
This research was supported by Veterans Administration Research Service.
Conflicts of interest
Dr. Laghi’s research laboratory has received a research grant from Liberate Medical LLC. Dr. Tobin receives royalties from McGraw-Hill for two books published on critical care medicine.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laghi, F., D’Alfonso, N. & Tobin, M.J. A paper on the pace of recovery from diaphragmatic fatigue and its unexpected dividends. Intensive Care Med 40, 1220–1226 (2014). https://doi.org/10.1007/s00134-014-3340-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-014-3340-6