Abstract
Purpose
The management of trauma patients suffering from active bleeding has improved with a better understanding of trauma-induced coagulopathy. The aim of this manuscript is to give recommendations for coagulation management.
Methods
A systematic literature search in the PubMed database was performed for articles published between January 2000 and August 2009. A total of 230 articles were included in the present systematic review.
Conclusions
The “coagulopathy of trauma” is a discrete disease which has a decisive influence on survival. Diagnosis and therapy of deranged coagulation should start immediately after admission to the emergency department. A specific protocol for massive transfusion should be introduced and continued. Loss of body temperature should be prevented and treated. Acidaemia should be prevented and treated by appropriate shock therapy. If massive transfusion is performed using fresh frozen plasma (FFP), a ratio of FFP to pRBC (packed red blood cells) of 1:2–1:1 should be achieved. Fibrinogen should be substituted at levels of <1.5 g/L. For patients suffering from active bleeding, permissive hypotension (i.e. mean arterial pressure ~65 mmHg) may be aimed for until surgical cessation of bleeding. This option is contraindicated in injuries of the central nervous system and in patients with coronary heart disease, or with known hypertension. Thrombelastography or -metry may be performed to guide coagulation diagnosis and substitution. Hypocalcaemia <0.9 mmol/L should be avoided and may be treated. For actively bleeding patients, pRBC may be given at haemoglobin <10 g/L (6.2 mmol/L) and haematocrit may be targeted at 30%.
Similar content being viewed by others
Introduction
Massive bleeding following traumatic injury remains the second most common cause of death in industrialised countries. For trauma patients dying in hospital, uncontrollable haemorrhage is the most frequent preventable cause of death [1]. This review discusses the state-of-the-art of coagulation management in actively bleeding trauma patients.
Methods
A systematic search in PubMed was conducted in August 2009 on the topics of “trauma and coagulation” as well as “therapeutic options”. All publications beginning in the year 2000 were considered. The PubMed search headings and some statistical details of the cited publications can be found online in the “Electronic supplementary material”. The query was limited to clinical trials, meta-analyses, randomized controlled trials (RCTs) and reviews. The results were compared and duplicates were removed. The remaining articles were sorted according to their relevance. For clarification purposes or missing publications, the limitation “starting 2000 and human” was abandoned. As Germany is the only country where the use of blood products is regulated by a special transfusion law requiring the publication of cross-sectional guidelines, the German Medical Association’s 4th revised edition of the Cross-sectional Guidelines for Therapy with Blood Components and Plasma Derivatives [2] was an additional focus of the article. Because of their great significance, two papers published in 2010 were included: CRASH-2 [3] and the European trauma guidelines [4]. After considering the publications’ references, we selected a total of 230 articles that formed the basis of our review. On the basis of the 2001 classification published by the Oxford Centre of Evidence Based Medicine (CEBM, www.cebm.net), an evaluation of the level of evidence (LoE) of the cited articles was consensually made by the authors. Almost half of these articles were downgraded as they were contextually not totally accurate. To evaluate the LoE of our core findings, the CEBM classification system using grade A (LoE 1), B (LoE 2–3) or 0 (LoE 4–5) was chosen. These preliminary recommendations were discussed at several meetings of the writing team, revised and finally approved in recommendations.
Results and discussion
The term “multiple trauma” refers to heterogeneous injury patterns. For decades, perioperative coagulation therapy was performed according to “gut instinct” and “expert opinions”. A growing number of experimental and clinical studies have been published based on scientific data. Although the pathophysiological context of the particular therapies and deranged coagulation is coherent and consistent, RCTs are lacking for almost all recommendations. Like the Cross-sectional Guidelines for Therapy with Blood Components and Plasma Derivatives, our recommendations are mostly based on case observations and non-randomized trials. Hence, only a grade of recommendation (GoR) “0” can be given for many. Therefore and because of the cost of the particular therapies, it must be emphasised that the options listed are indicated only for patients with massive, life-threatening haemorrhage [5].
Acute coagulopathy of trauma
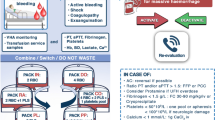
Bleeding in massively injured patients increases with additional coagulopathy [6]. Coagulopathy following trauma was assumed to be secondary, i.e. caused by loss/dilution and amplified by acidaemia/hypothermia (“lethal triad”, “bloody vicious circle”) [7]. Recent literature has proved the existence of a distinct, multifactorial, primary disorder, secondarily amplified by consumption, loss and dilution [8, 9]. Acute coagulopathy of trauma (ACoT) significantly impairs survival [10], correlates independently with an eightfold increase in mortality within 24 h [9] and a quadrupled total mortality [10]. Patients arriving at the emergency department (ED) coagulopathic stay longer in the intensive care unit (ICU) and hospital, have an increased risk for renal insufficiency and multiple organ failure, need longer ventilatory support and show a tendency towards increased lung injury [9, 11]. Tissue trauma causes hypoperfusion and hyperfibrinolysis [12]. The intensity of hyperfibrinolysis correlates with trauma severity [13]. Some 30% of the massively injured patients show ACoT upon arrival in the ED [8–10, 14]. The exact mechanism of ACoT is still unclear; Brohi [11] published a possible pathophysiological mechanism focusing on activation of the protein C pathway and hypoperfusion (Fig. 1). There is no intravascular thrombosis in the early phase of trauma; the definition of disseminated intravascular coagulation (DIC) does not apply in the case of ACoT [15]. Some authors do think ACoT and DIC are related and contradict Brohi’s explanations [16].
Hypoperfusion causes increased endothelial expression of thrombomodulin. The thrombin–thrombomodulin complex activates protein C, which (together with protein S) inhibits the accelerators FVa and FVIIa. Additionally, larger quantities of aPC decrease plasminogen activator inhibitor, the most essential antagonist for tissue plasminogen activator. Together with decreased plasmin levels, hyperfibrinolysis is triggered (modified from [11]). Thr thrombin, Tm thrombomodulin, aPC activated protein C, PS protein S, PAI 1 plasminogen activator inhibitor 1, t-PA tissue plasminogen activator
Recommendation The “coagulopathy of trauma” is a discrete disease which has decisive influence on survival. Diagnosis and therapy of deranged coagulation should start immediately in the ED (GoR A).
Diagnostics
ACoT presents clinically with non-surgical, diffuse bleeding from mucosal or damaged tissue, new bleeding from vesical/gastric tubes or from puncture sites. Coagulation screening tests such as prothrombin time (PT) or activated partial thromboplastin time (aPTT) are weak predictors of bleeding tendencies in the critically ill [17]. These tests measure only the first 20–60 s of clot formation; a process which is probably not complete for 15–30 min.
Routine laboratory tests cannot assess the effect of hypothermia, acidosis, hypocalcaemia or anaemia on haemostasis [18]. Abnormal coagulation tests are increasingly frequent with increasing injury severity [19]. Trauma registers have demonstrated that an increased PT correlates with significantly higher mortality [10, 19]. PT/aPTT alone should not be used to guide haemostatic therapy, whereas serum lactate and base deficit are sensitive tests to monitor the extent of bleeding and shock [4].
Recommendation Diagnosis of ACoT by “classical” coagulation parameters like PT, aPTT or platelet count is insufficient. Yet, no better parameter is available (GoR 0).
Thrombelastography/-metry
Coagulation monitoring in massively injured patients by means of thrombelastography (TEG) or -metry (ROTEM) has been increasingly analysed. These devices not only measure the start of clot formation, but also its velocity, maximal amplitude and lysis. A number of TEG- or ROTEM-based algorithms have been published for this setting [20], enabling faster therapeutic decisions [8, 21].
TEG differentiated the mechanism related to clotting abnormalities in porcine models and may permit focused treatment of clotting alterations associated with hypothermia and haemorrhagic shock [22]. With a sensitivity and specificity between 74 and 100%, ROTEM rapidly detects systemic changes of in vivo coagulation, in particular trauma-induced hyperfibrinolysis [8, 13]. In 33 severely injured patients, ROTEM-based diagnosis of hyperfibrinolysis predicted outcome: fulminant (within 30 min), intermediate (30–60 min) or late hyperfibrinolysis resulted in 100, 91 or 73% mortality, respectively. Results suggested a disproportionally higher incidence of hyperfibrinolysis in injuries with increasing severity [23].
The implementation of TEG or ROTEM for trauma coagulation management is promising, particularly with regard to hyperfibrinolysis [2], and suggested by the recently published European trauma guidelines [4]. Further prospective evaluation and an evidence-based determination of the parameters’ cut-off levels are required [5, 24].
Recommendation ROTEM/TEG may be performed to guide coagulation diagnosis and substitution (GoR 0).
“Damage control resuscitation”
“Damage control surgery” delays definitive anatomical therapy in favour of stabilising vital physiology. Damage control resuscitation (DCR) deals with trauma-induced coagulopathy [25]. This concept includes permissive hypotension, prevention of acidaemia, hypocalcaemia and hypothermia, as well as haemostatic resuscitation [4, 24].
Permissive hypotension
This approach consists of targeting a lower than normal blood pressure to support clot formation, and infusing fluids sparsely while maintaining sufficient perfusion. Clinical use of this concept is based on Bickell’s publication [26]: in 598 adult patients with penetrating torso injuries and a prehospital systolic blood pressure (SBP) of ≤90 mmHg, the delay of aggressive fluid resuscitation until surgical intervention improved outcome (shortcomings in study design, retrospective analysis, conduction and interpretation [27]). Data from the German Trauma Registry proved that increased prehospital fluids cause increased coagulopathies [9]. Prehospital intravenous fluid replacement therapy in serious trauma effected no advantage or disadvantage [28]. Titration of initial fluid therapy to an SBP of 70 mmHg during active haemorrhage did not affect mortality [29]. A Cochrane review found no evidence from RCTs for or against early or larger volumes of intravenous fluid administration in uncontrolled haemorrhage [30].
Recommendation Despite a lack of evidence-based proof and based on pathophysiological considerations, for actively bleeding patients, permissive hypotension, i.e. mean arterial pressure (MAP) ~65 mmHg or SBP ~90 mmHg, may be targeted until surgical cessation of bleeding. This option is contraindicated in injuries of the central nervous system, coronary heart disease, or hypertension [4, 31] (GoR 0).
(Re)warming
Hypothermia ≤34°C notably impairs platelet function and coagulation factor activity [18]. Initial fluid therapy should be carried out with warmed infusions [25, 32] and any volume therapy only via a warming device with a fluid temperature of 40–42°C [32]. Passive (insulating foil, blankets, removal of wet clothes) and active (fluid warmers, heated air, heat radiation) measures are needed. Room temperature should be raised in the ED as well as in the operating room, at best to a thermally neutral range (28–29°C) [4].
Desmopressin partially reverses hypothermia-induced impairment of primary haemostasis in vitro [33]. RCTs in trauma are lacking.
Recommendation Loss of body temperature should be prevented and treated by appropriate means to achieve and maintain normothermia [4] (GoR B).
Reduction of acidaemia
A pH ≤7.2 impairs coagulation [2, 18]. Because the main cause of acidosis is hypoperfusion, it will persist until restoration of sufficient tissue perfusion. The base excess (BE) affects coagulation [18], and is a prognostic factor for complications and death [4]. The critical range for BE begins between −6 and −10 mmol/L [34]. Massive transfusion of packed red blood cells (pRBC) further increases the acid load [35].
Buffering does not stop coagulopathy [36] and is recommended only in combination with administration of drugs affecting coagulation.
Recommendation Acidaemia should be prevented and treated by appropriate shock therapy (GoR B).
Correction of ionised calcium
A reduction in ionised calcium following transfusion is caused by the anticoagulant citrate and is particularly marked when using fresh frozen plasma (FFP). The faster the transfusion is given, the faster the reduction occurs [2].
Recommendation Hypocalcaemia <0.9 mmol/L should be avoided and may be treated [2, 4, 18] (GoR 0).
Replacement therapy for coagulation
Packed red blood cells
A growing number of publications demonstrate the detrimental effects of RBC transfusion on patient survival [25]. However, the involvement of RBC in coagulation is proven [18, 37]. Restrictive transfusion triggers in coagulopathic patients may be detrimental [38], as affected haemostasis appears long before impaired oxygenation [37].
Recommendation Because of the beneficial effects of higher haematocrit (Hct) levels on primary haemostasis, for actively bleeding patients, RBC may be given at haemoglobin (Hb) <10 g/L (6.2 mmol/L) and haematocrit may be targeted at 30% [2, 4] (GoR 0).
Fresh frozen plasma
In a systematic review, Stanworth [39] found little evidence that FFP is an effective treatment for massive transfusions. Infusing only 10 mL/kg body weight (BW) caused a plasma dilution of 21% for almost 3 h. Sufficient increases in coagulation factors demanded 30 mL/kgBW or an average of 2.5 L of FFP [17].
Although transfusion of any blood product can cause transfusion-related acute lung injury (TRALI), it is most often induced by FFP [40]. An FFP/RBC ratio >1:1.5 caused an almost doubled risk of TRALI [41]. A significant association was found between transfusion of FFP and ventilator-associated pneumonia (VAP) with shock, VAP without shock, bloodstream infection with shock, and undifferentiated septic shock in surgical patients [42]. The relative risk (RR) for transfusion of FFP and all infections was 2.99. The magnitude of risk of infection associated with each unit of FFP was approximately 4%. In 14,070 trauma patients FFP transfusion was an independent predictor of ALI: RR after 1–5 FFP of 1.66 and with >5 FFP of 2.55 [43].
Recommendation In ongoing, severe bleeding, sole coagulation substitution by FFP may not be sufficient (GoR 0).
FFP/RBC ratio
Even if the use FFP increases the risks of pulmonary and bloodstream infections and prospective trials are missing, there is growing evidence that a high FFP/RBC ratio may be beneficial for patients with life-threatening haemorrhage: Instituting a civilian massive transfusion protocol (FFP/pRBC/platelets 1:1:1) led to a drastic reduction in early coagulopathy, in mortality within 24 h, and mortality within 30 days for blunt trauma [44]. A 4-year retrospective study with 135 trauma patients reported that an FFP/pRBC ratio of 1:1 conferred a survival advantage in patients requiring massive transfusion [45]. Comparing an ED transfusion protocol (FFP/pRBC <1:2) with that of an ICU (FFP/pRBC 1:1) showed that coagulopathy present at hospital admission persisted at ICU admission, and severity of coagulopathy at ICU admission was associated with survival outcome [46]. A significantly reduced 30-day mortality for massively transfused patients with ≥2:3 was noted [47]. Holcomb [48] examined records of 467 massively transfused civilian trauma patients: FFP/pRBC ≥1:2 made for a better 30-day survival. The combination of high plasma and high platelet to RBC ratios was associated with increased 6-h, 24-h and 30-day survival, as well as increased ICU-, ventilator- and hospital-free days. A total of 133 patients with >10pRBC within 6 h revealed: Median FFP/RBC in survivors was 1:2, in non-survivors 1:4. The highest survival rate was for a ratio between 1:2 and 1:3, whereas 1:1 led to increased mortality [49]. 713 patients had a survival benefit depending on an FFP/pRBC favouring >1:1. A ratio >1:1 caused more ventilator days and longer ICU stays [50]. A 24-h survival benefit accompanied by an increased risk of TRALI was found in 415 patients with FFP/pRBC >1:1.5 [41]. Scalea [51] prospectively noted no significant difference in outcome when comparing patients who had a 1:1 FFP/RBC ratio with those who did not receive any FFP. The authors concluded that with an overall mortality of 4% within 24 h (6% for the 81 patients with massive transfusion), their study examined different patients than most other publications. A linear survival benefit with increasing FFP/pRBC towards 1:3 (excluding severe head injuries) was published: FFP/pRBC was the second most important predictor of outcome after admission GCS [52]. Snyder et al. [53] retrospectively conducted an outcome study of 134 patients requiring ≥10 units (U) of pRBC/24 h. The authors noted a significantly reduced 24-h mortality of 40% at a ratio of FFP/pRBC ≥1:2 (median 1:1.3) compared with 58% with <1:2 (median 1:3.7). This significance was no longer detectable if the exact time of transfusion was considered. The authors proposed a “survival bias”: the non-survivors did not die because they received less FFP, but rather received less because they died. Reviewing 66 randomised, massive transfusion trauma patients (≥10 U of pRBC/24 h) at 16 level-1 trauma centres higher 6-h FFP/pRBC and PLT/pRBC ratios lead to improved 6-h and in-hospital mortality [54].
Recommendation If massive transfusion is performed using FFP, an FFP/RBC ratio of 1:2–1:1 should be targeted (GoR B).
Massive transfusion protocol
The term “massive transfusion” (MT) is mostly used for ≥10 U pRBC within 24 h [24], alternatively ≥10 U pRBC within 6 h [49]. The introduction of a massive transfusion protocol (MTP) with an FFP/pRBC of 1:1.5 resulted in a 74% reduction in the odds of mortality, and a higher 30-day survival, with a reduction in hospitalisation time and ventilator days. The authors concluded than an MTP delivered in an aggressive manner significantly reduces the risk of mortality as well as overall blood product consumption [55]. Using an MTP improved 24-h and 30-day survival and caused fewer deaths from coagulopathy [44].
Recommendation A specific protocol for massive transfusion should be introduced and continued (GoR B).
Platelets
In initial acute loss, the bone marrow and spleen increasingly release platelets (PLT). They decrease late in first-time bleeding. Following transfusion, the PLT disperse in the blood and spleen; the recovery rate in peripheral blood amounts to only 60–70%, or even less in conditions with increased PLT consumption [2].
A retrospective multi-centre study found an increased 30-day survival with PLT/pRBC ≥1:2. PLT/RBC ratios and injury severity score were predictors of death at 6 h, 24 h and 30 days [48]. A multi-centre study of MT within 6 h found that PLT/pRBC ≥1:1 produced significant improvements [54].
Recommendation For acute haemorrhage platelet transfusion is recommended in the case of transfusion-dependent bleeding if platelet counts are <100,000 μL−1 [2, 4] (GoR 0).
Fibrinogen
Factor I (fibrinogen) is the substrate of plasmatic coagulation. It is essential for the formation of the fibrin net and, as a ligand for GPIIb-IIIa receptors on the platelet surface, for aggregation. Fibrinogen levels are the first procoagulatory factors to decline while other clotting factors and platelets critically drop in cases of much more extensive blood loss [2]. PT and aPTT demonstrate pathological values only in pronounced fibrinogen deficiency below 1 g/L [2]. The mean dose is around 3–5 g [2] or 50 mg/kgBW.
In vitro dilution by infusing crystalloids or colloids and particularly hydroxyethyl starch (HES) seems to impair the interaction of thrombin, factor XIII and fibrinogen [56]. Substituting fibrinogen (~3 g/70 kgBW) led to an improvement in ROTEM parameters [57]. Impaired clot formation during thrombocytopenia improved with administration of fibrinogen concentrate in a porcine model (reduced blood loss, prolonged survival) [58]. In patients with combat-related trauma requiring massive transfusion, the transfusion of an increased fibrinogen to RBC ratio (>1 g/5 U pRBC) was independently associated with decreasing death from haemorrhage [59]. Substitution therapy with a fibrinogen concentrate generally improved global laboratory coagulation results and appeared to reduce blood loss. Requirements for RBC, FFP and platelet substitution also diminished during serious haemorrhage [60]. Purified, virally inactivated fibrinogen concentrate was effective in the management of 30 adult patients who received fibrinogen concentrate for acquired hypofibrinogenaemia (<1.5 g/L) and enabled rapid administration of a median fibrinogen dose of 4 g in an emergency setting [61]. The administration of fibrinogen concentrates in unresponsive, life-threatening haemorrhage with acquired hypofibrinogenaemia improved laboratory measures of coagulation and significantly improved 7-day survival. Observational data indicated a direct relationship between plasma fibrinogen levels and survival in acquired fibrinogen deficiency [62].
The results of indirect fibrinogen assays (derived fibrinogen and Clauss’ method) should be interpreted cautiously when colloids are used [63].
Recommendation In cases of acute bleeding or relevant disposition to bleeding, one should aim for a level >1.5 g/L and determine fibrinogen concentrations with the method according to Clauss (≥2 g/L if colloids were infused) [2, 4] (GoR B).
Prothrombin complex concentrates
Factors II, VII, IX and X of the prothrombin complex as well as the proteins C, S and Z are standardised with respect to their content in factor IX [2]. Adverse reactions such as thromboembolic episodes, DIC and/or hyperfibrinolysis are extremely unlikely, even when larger doses are administered [2]. No general antithrombin substitution is required [2].
The main reasons for administering prothrombin complex concentrates (PCCs) are to help ensure viral safety during use of plasma products and to minimise the risk of TRALI. In coagulopathy, the prothrombin complex deficiency may be pronounced to such a degree that (in addition to FFP infusion) substitution with PCC is required [2]. Substitution of fibrinogen and PCC after a standardized liver cut in a porcine dilution model using 6% HES 130/0.4 caused significant reduced blood loss and survival of all pigs whereas 80% of controls died [64]. PCC shortened the time to haemostasis after either bone or spleen trauma in animals and reduced the volume of blood lost. Subsequent to bone injury, PCC versus 40 mL/kg FFP also accelerated haemostasis and diminished blood loss; substitution with PCC effectively normalized coagulation and significantly improved haemostasis and blood loss [65].
Recommendation In acquired deficiencies, the application of PCC (initially 1,200–2,400 IU or 20–25 IU/kgBW) may help to stop the bleeding (GoR 0).
Antifibrinolytics
Hyperfibrinolysis in trauma appears to be more frequent than previously assumed [66] and its intensity correlates with injury severity [13]. It is most common in patients with chest, blunt abdominal, pelvic, or head trauma. Damage to inhibitory mechanisms or an excessive release of plasmin shift the coagulation system towards increased fibrinolysis with massive bleeding. Antifibrinolytic treatment of ACoT is logically based on the mechanism proposed by Brohi [11]. ROTEM provided rapid and accurate detection of hyperfibrinolysis in severely injured trauma patients [4, 13, 23]. The administration of fibrinogen is only indicated following interruption of the coagulation disorder by antifibrinolytics [2]. A 2004 Cochrane review found insufficient evidence from RCTs of antifibrinolytics in trauma to either support or refute a clinically important treatment effect [67]. In the double-blind, randomised, multi-centre and multinational CRASH-2 trial [3] 10,060 adult trauma patients with or at risk of severe haemorrhage received 1 g of tranexamic acid (TxA) in 10 min plus 1 g over 8 h, and 10,067 matched patients received 0.9% saline. TxA significantly reduced all-cause mortality and mortality due to bleeding. Vascular occlusive events did not differ significantly.
Recommendation TxA (1–2 g or 1 g in 10 min plus 1 g over 8 h) should be considered for use in bleeding trauma patients (GoR B).
Desmopressin
Desmopressin (DDAVP) enhances platelet adhesion to the vessel wall and increases factor VIII (FVIII) and von Willebrand factor (VWF) plasma concentrations [68]. The clinical indications for DDAVP have rapidly expanded beyond haemophilia and von Willebrand disease (thrombocytopenia, uraemia, hepatic cirrhosis, antiplatelet drugs) [69]. The maximal effect occurs after 90 min [68]. Repetitive doses may release tissue plasminogen activator (tPA) and thus may cause hyperfibrinolysis. Some authors recommend that repeated administrations should be accompanied by TxA [68]. Trials with DDAVP in cardiac surgery reported a non-significantly elevated incidence of myocardial infarct in the DDAVP-treated group [68]. Desmopressin partially reverses hypothermia-induced impairment of primary haemostasis in vitro [33]. A Cochrane review [70] found no convincing evidence that DDAVP minimises perioperative RBC transfusion in patients who do not have congenital bleeding disorders. Zotz [71] reviewed the same data for patients with blood loss >1 L or a history of aspirin use. This therapeutic use of DDAVP caused a significant reduction in blood loss and transfused RBC. There are no RCTs for trauma patients.
Recommendation In diffusely bleeding patients with suspected thrombocytopathy, intravenous DDAVP administration at a dose of 0.3 mg/kg diluted in 50 mL saline, infused over 30 min, may be considered (GoR 0).
Factor XIII
Activated factor XIII (FXIII) is a transglutaminase which cross-links fibrin covalently in the presence of calcium ions. A firm three-dimensional fibrin net is formed resulting in definite haemostasis. FXIII is not determined by the screening tests PT and aPTT [2].
Acquired FXIII deficiency may be caused by increased turnover (e.g. following intravascular clotting, increased blood loss, hyperfibrinolysis) or by increased consumption (e.g. in major surgery) [2]. Perioperatively this may lead to massive bleeding [72]. For intracranial and cardiac surgery, the association of decreased perioperative FXIII with an increased risk of postoperative haematoma/bleeding was proven. Examination of the occurrence of unexplained intraoperative bleeding in elective surgery revealed that fibrinogen and FXIII were more rapidly consumed in bleeders. ROTEM showed a significant reduction in clot firmness. FXIII substitution (30 U/kg) in surgical cancer patients at high risk for intraoperative bleeding led to significantly reduced loss of clot firmness and a trend towards reduced blood loss [72]. RCTs in the trauma setting are lacking.
Recommendation In case a prompt determination of FXIII is not possible, the risk of persistent bleeding should be weighed against that of blind administration of FXIII, particularly in acute severe haemorrhage. In cases of bleeding, at least 15–20 U/kgBW may be substituted until haemostasis is achieved [2] (GoR 0).
Recombinant activated factor VII
Supraphysiological doses of recombinant activated factor VII (rFVIIa) cause a maximum number of tissue factor molecules to form complexes and activate the clotting system limited to the site of injury. A pathway for the initiation of coagulation is formed that is independent of sufficient activation of FIX, FVIII or tissue factor [73]. Because of the lower affinity of FVIIa to activated platelets, a supraphysiological dose is necessary to achieve haemostasis. FVIIa has almost no affinity at all to inactive platelets [2, 74].
Off-label use of rFVIIa has been published in numerous case reports, cohorts and some RCTs. Thromboembolic events have occurred in the arterial and venous vascular system, in particular in patients receiving rFVIIa off-label [2]. Early use of rFVIIa (within 2.5 h of trauma) was associated with decreased 24-h and 30-day mortality in severely injured combat casualties requiring massive transfusion [75]. In the only positive class-1 study, Boffard [76] tested rFVIIa as an adjunctive therapy for bleeding control in severely injured trauma patients with 400 μg/kgBW. This resulted in a significant reduction in RBC transfusion and the need for massive transfusion in severe blunt trauma. A trend was seen for penetrating trauma, mortality and critical complications; no increased adverse events were observed. When patients who required admission to the ICU were analysed (n = 110, 50% received rFVIIa), total mean charges and costs were significantly lower in the group that received rFVIIa [77]. Hospital length of stay (LOS), days of mechanical ventilation and plasma utilization were lower in the rFVIIa group. However, the authors administered relatively small doses of rFVIIa (mean 41.9 ± 35.5 μg/kg, median 25.1 μg/kg). For off-label use, rFVIIa’s effectiveness remains uncertain—either prophylactic or therapeutically [78]. In 22 RCTs with 3,184 patients (the only trauma patients were those of Boffard [76]), those receiving rFVIIa were less likely to need additional blood transfusions. Reduction in mortality was more likely if rFVIIa was given therapeutically. The incidence of arterial thromboemboly was higher in patients receiving rFVIIa. Nineteen RCTs focused on trauma: based on Boffard’s publication [76] the authors gave a “level 1” recommendation for the use of rFVIIa in blunt trauma, and a “level 2” for halting trauma-associated haemorrhage when other therapies have failed with 400 μg/kg (a lower dosage may be as efficacious) [79]. A review of prospective, controlled trials that involved the therapeutic use of rFVIIa in the ED found no significant difference in mortality between rFVIIa and placebo [80]. A large multinational phase lll trial of rFVlla in trauma patients was recently terminated due to futility, because the planned reduction in mortality could not be achieved [24].
The effect of rFVIIa depends on several concomitant factors whose maintenance is paramount: fibrinogen ≥1 g dL−1, haemoglobin ≥7 g/dL, platelets ≥50,000 μL−1 (better ≥100,000), Ca2+ ≥0.9 mmol/L, temperature ≥34°C, pH ≥7.2, and the exclusion of hyperfibrinolysis or heparin [2, 74, 81]. A common “standard dose” for off-label use is 90 μg/kgBW [73, 82]. However, the required dose is still under debate [81]. A repetitive dose may be needed within 30 min–2 h, although repeat dosing is more likely to indicate futility than to be effective [82].
The review by Mannucci [83] summarises: “This agent is not a panacea, but it has efficacy in patients with trauma and excessive bleeding that is resistant to other treatments.” Administration only if other (conventional) therapeutic options have not been effective is also proposed in current reviews [79, 82, 84]. Since 2009, NovoNordisk no longer recommends the off-label use of rFVIIa due to a doubling in the risk of (arterial) thrombosis between 1/10 and 1/100 (see German prescription recommendations dated May 2009, and NovoNordisk’s statement: “Safety and effectiveness has not been established in these settings and the use is not approved by FDA”).
Recommendation For patients with traumatic haemorrhage rFVIIa may be used on a case-by-case basis, if other therapeutic options failed, and after consideration and correction of concomitant factors, especially in blunt trauma [4] (GoR B).
Antithrombin
Antithrombin (AT) deficiency in ACoT is not isolated as all pro- and anticoagulants undergo consumption [4]. The administration of AT in ongoing massive haemorrhage will increase bleeding and is not recommended [4]. In critically ill patients, AT increased the risk of bleeding events and failed to reduce overall mortality [85].
Recommendation Actively bleeding patients should not be treated with AT [4] (GoR B).
A summary of the above-mentioned therapeutic options in trauma-induced haemorrhage is presented in Table 1.
Conclusion
Recent scientific work enables a new view of the old problem of ACoT: traumatic coagulopathy is a distinct disease with hyperfibrinolysis as a (possible) key element. For life-threatening haemorrhage, sole application of FFP may not be sufficient. Early, aggressive, anticipatory and empirical (or better ROTEM-/TEG-guided) therapy with pRBC, FFP and purified factor concentrates together with consideration and correction of concomitant factors (temperature, pH, ionised calcium) will help rescue more of our patients.
References
Hess JR, Dutton RB, Holcomb JB, Scalea TM (2008) Giving plasma at a 1:1 ratio with red cells in resuscitation: who might benefit? Transfusion 48:1763–1765
Bundesärztekammer BÄK (German Medical Association) (2009) Cross-sectional guidelines for therapy with blood components and plasma derivatives—4th edn. http://www.bundesaerztekammer.de/downloads/LeitCrossBloodComponents4ed.pdf
Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, El-Sayed H, Gogichaishvili T, Gupta S, Herrera J, Hunt B, Iribhogbe P, Izurieta M, Khamis H, Komolafe E, Marrero MA, Mejia-Mantilla J, Miranda J, Morales C, Olaomi O, Olldashi F, Perel P, Peto R, Ramana PV, Ravi RR, Yutthakasemsunt S (2010) Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 376:23–32
Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, Hunt BJ, Komadina R, Nardi G, Neugebauer E, Ozier Y, Riddez L, Schultz A, Stahel PF, Vincent JL, Spahn DR (2010) Management of bleeding following major trauma: an updated European guideline. Crit Care 14:R52
Lier H, Krep H, Schochl H (2009) Coagulation management in the treatment of multiple trauma. Anaesthesist 58:1010–1026
Shaz BH, Dente CJ, Harris RS, MacLeod JB, Hillyer CD (2009) Transfusion management of trauma patients. Anesthesiol Analg 108:1760–1768
Hoyt DB, Bulger EM, Knudson MM, Morris J, Ierardi R, Sugerman HJ, Shackford SR, Landercasper J, Winchell RJ, Jurkovich G (1994) Death in the operating room: an analysis of a multi-center experience. J Trauma 37:426–432
Rugeri L, Levrat A, David JS, Delecroix E, Floccard B, Gros A, Allaouchiche B, Negrier C (2007) Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography. J Thromb Haemost 5:289–295
Maegele M, Lefering R, Yucel N, Tjardes T, Rixen D, Paffrath T, Simanski C, Neugebauer E, Bouillon B (2007) Early coagulopathy in multiple injury: an analysis from the German Trauma Registry on 8,724 patients. Injury 38:298–304
MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M (2003) Early coagulopathy predicts mortality in trauma. J Trauma 55:39–44
Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet JF (2007) Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg 245:812–818
Hess JR, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, Kluger Y, Mackway-Jones K, Parr MJ, Rizoli SB, Yukioka T, Hoyt DB, Bouillon B (2008) The coagulopathy of trauma: a review of mechanisms. J Trauma 65:748–754
Levrat A, Gros A, Rugeri L, Inaba K, Floccard B, Negrier C, David JS (2008) Evaluation of rotation thrombelastography for the diagnosis of hyperfibrinolysis in trauma patients. Br J Anaesth 100:792–797
Brohi K, Singh J, Heron M, Coats T (2003) Acute traumatic coagulopathy. J Trauma 54:1127–1130
Hess JR, Lawson JH (2006) The coagulopathy of trauma versus disseminated intravascular coagulation. J Trauma 60:S12–S19
Gando S (2009) Acute coagulopathy of trauma shock and coagulopathy of trauma: a rebuttal. You are now going down the wrong path. J Trauma 67:381–383
Chowdhury P, Saayman AG, Paulus U, Findlay GP, Collins PW (2004) Efficacy of standard dose and 30 ml/kg fresh frozen plasma in correcting laboratory parameters of haemostasis in critically ill patients. Br J Haematol 125:69–73
Lier H, Krep H, Schroeder S, Stuber F (2008) Preconditions of hemostasis in trauma: a review. The influence of acidosis, hypocalcemia, anemia, and hypothermia on functional hemostasis in trauma. J Trauma 65:951–960
Hess JR, Lindell AL, Stansbury LG, Dutton RP, Scalea TM (2009) The prevalence of abnormal results of conventional coagulation tests on admission to a trauma center. Transfusion 49:34–39
Kozek-Langenecker S (2007) Monitoring hemostasis in emergency medicine. In: Vincent JL (ed) Yearbook of intensive care emergency medicine 2007, vol XXXII, Springer, Berlin, pp 847–859
Plotkin AJ, Wade CE, Jenkins DH, Smith KA, Noe JC, Park MS, Perkins JG, Holcomb JB (2008) A reduction in clot formation rate and strength assessed by thrombelastography is indicative of transfusion requirements in patients with penetrating injuries. J Trauma 64:S64–S68
Martini WZ, Cortez DS, Dubick MA, Park MS, Holcomb JB (2008) Thrombelastography is better than PT, aPTT, and activated clotting time in detecting clinically relevant clotting abnormalities after hypothermia, hemorrhagic shock and resuscitation in pigs. J Trauma 65:535–543
Schöchl H, Frietsch T, Pavelka M, Jambor C (2009) Hyperfibrinolysis after major trauma: differential diagnosis of lysis patterns and prognostic value of thrombelastometry. J Trauma 67:125–131
Spinella PC, Holcomb JB (2009) Resuscitation and transfusion principles for traumatic hemorrhagic shock. Blood Rev 23:231–240
Beekley AC (2008) Damage control resuscitation: a sensible approach to the exsanguinating surgical patient. Crit Care Med 36:S267–S274
Bickell WH, Wall MJ Jr, Pepe PE, Martin RR, Ginger VF, Allen MK, Mattox KL (1994) Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med 331:1105–1109
Jacobs LM (1994) Timing of fluid resuscitation in trauma. N Engl J Med 331:1153–1154
Turner J, Nicholl J, Webber L, Cox H, Dixon S, Yates D (2000) A randomised controlled trial of prehospital intravenous fluid replacement therapy in serious trauma. Health Technol Assess 4:1–57
Dutton RP, Mackenzie CF, Scalea TM (2002) Hypotensive resuscitation during active hemorrhage: impact on in-hospital mortality. J Trauma 52:1141–1146
Kwan I, Bunn F, Roberts I (2003) Timing and volume of fluid administration for patients with bleeding. Cochrane Database Syst Rev CD002245
Theusinger OM, Spahn DR, Ganter MT (2009) Transfusion in trauma: why and how should we change our current practice? Curr Opin Anaesthesiol 22:305–312
Tieu BH, Holcomb JB, Schreiber MA (2007) Coagulopathy: its pathophysiology and treatment in the injured patient. World J Surg 31:1055–1064
Ying CL, Tsang SF, Ng KF (2008) The potential use of desmopressin to correct hypothermia-induced impairment of primary haemostasis? An in vitro study using PFA-100[(R)]. Resuscitation 76:129–133
Yucel N, Lefering R, Maegele M, Vorweg M, Tjardes T, Ruchholtz S, Neugebauer EA, Wappler F, Bouillon B, Rixen D (2006) Trauma associated severe hemorrhage (TASH)-score: probability of mass transfusion as surrogate for life threatening hemorrhage after multiple trauma. J Trauma 60:1228–1236
Spahn DR, Rossaint R (2005) Coagulopathy and blood component transfusion in trauma. Br J Anaesthiol 95:130–139
Brohi K, Cohen MJ, Davenport RA (2007) Acute coagulopathy of trauma: mechanism, identification and effect. Curr Opin Crit Care 13:680–685
Hardy JF, De MP, Samama M (2004) Massive transfusion and coagulopathy: pathophysiology and implications for clinical management. Can J Anaesth 51:293–310
McDonald V, Ryland K (2008) Coagulopathy in trauma: optimising haematological status. Trauma 10:109–123
Stanworth SJ, Brunskill SJ, Hyde CJ, McClelland DB, Murphy MF (2004) Is fresh frozen plasma clinically effective? A systematic review of randomized controlled trials. Br J Anaesth 126:139–152
Toy P, Lowell C (2007) TRALI–definition, mechanisms, incidence and clinical relevance. Best Pract Res Clin Anaesthesiol 21:183–193
Sperry JL, Ochoa JB, Gunn SR, Alarcon LH, Minei JP, Cuschieri J, Rosengart MR, Maier RV, Billiar TR, Peitzman AB, Moore EE (2008) An FFP:PRBC transfusion ratio ≥1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma 65:986–993
Sarani B, Dunkman WJ, Dean L, Sonnad S, Rohrbach JI, Gracias VH (2008) Transfusion of fresh frozen plasma in critically ill surgical patients is associated with an increased risk of infection. Crit Care Med 36:1114–1118
Chaiwat O, Lang JD, Vavilala MS, Wang J, MacKenzie EJ, Jurkovich GJ, Rivara FP (2009) Early packed red blood cell transfusion and acute respiratory distress syndrome after trauma. Anesthesiology 110:351–360
Dente CJ, Shaz BH, Nicholas JM, Harris RS, Wyrzykowski AD, Patel S, Shah A, Vercruysse GA, Feliciano DV, Rozycki GS, Salomone JP, Ingram WL (2009) Improvements in early mortality and coagulopathy are sustained better in patients with blunt trauma after institution of a massive transfusion protocol in a civilian level I trauma center. J Trauma 66:1616–1624
Duchesne JC, Hunt JP, Wahl G, Marr AB, Wang YZ, Weintraub SE, Wright MJ, McSwain NE Jr (2008) Review of current blood transfusions strategies in a mature level I trauma center: were we wrong for the last 60 years? J Trauma 65:272–276
Gonzalez EA, Moore FA, Holcomb JB, Miller CC, Kozar RA, Todd SR, Cocanour CS, Balldin BC, McKinley BA (2007) Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma 62:112–119
Gunter OL Jr, Au BK, Isbell JM, Mowery NT, Young PP, Cotton BA (2008) Optimizing outcomes in damage control resuscitation: identifying blood product ratios associated with improved survival. J Trauma 65:527–534
Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, Gonzalez EA, Pomper GJ, Perkins JG, Spinella PC, Williams KL, Park MS (2008) Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg 248:447–458
Kashuk JL, Moore EE, Johnson JL, Haenel J, Wilson M, Moore JB, Cothren CC, Biffl WL, Banerjee A, Sauaia A (2008) Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma 65:261–270
Maegele M, Lefering R, Paffrath T, Tjardes T, Simanski C, Bouillon B (2008) Red-blood-cell to plasma ratios transfused during massive transfusion are associated with mortality in severe multiple injury: a retrospective analysis from the Trauma Registry of the Deutsche Gesellschaft fur Unfallchirurgie. Vox Sang 95:112–119
Scalea TM, Bochicchio KM, Lumpkins K, Hess JR, Dutton R, Pyle A, Bochicchio GV (2008) Early aggressive use of fresh frozen plasma does not improve outcome in critically injured trauma patients. Ann Surg 248:578–584
Teixeira PG, Inaba K, Shulman I, Salim A, Demetriades D, Brown C, Browder T, Green D, Rhee P (2009) Impact of plasma transfusion in massively transfused trauma patients. J Trauma 66:693–697
Snyder CW, Weinberg JA, McGwin G Jr, Melton SM, George RL, Reiff DA, Cross JM, Hubbard-Brown J, Rue LW III, Kerby JD (2009) The relationship of blood product ratio to mortality: survival benefit or survival bias? J Trauma 66:358–362
Zink KA, Sambasivan CN, Holcomb JB, Chisholm G, Schreiber MA (2009) A high ratio of plasma and platelets to packed red blood cells in the first 6 h of massive transfusion improves outcomes in a large multicenter study. Am J Surg 197:565–570
Cotton BA, Au BK, Nunez TC, Gunter OL, Robertson AM, Young PP (2009) Predefined massive transfusion protocols are associated with a reduction in organ failure and postinjury complications. J Trauma 66:41–48
Nielsen VG (2005) Colloids decrease clot propagation and strength: role of factor XIII-fibrin polymer and thrombin–fibrinogen interactions. Acta Anaesthesiol Scand 49:1163–1171
Fries D, Innerhofer P, Reif C, Streif W, Klingler A, Schobersberger W, Velik-Salchner C, Friesenecker B (2006) The effect of fibrinogen substitution on reversal of dilutional coagulopathy: an in vitro model. Anesth Analg 102:347–351
Velik-Salchner C, Haas T, Innerhofer P, Streif W, Nussbaumer W, Klingler A, Klima G, Martinowitz U, Fries D (2007) The effect of fibrinogen concentrate on thrombocytopenia. J Thromb Haemost 5:1019–1025
Stinger HK, Spinella PC, Perkins JG, Grathwohl KW, Salinas J, Martini WZ, Hess JR, Dubick MA, Simon CD, Beekley AC, Wolf SE, Wade CE, Holcomb JB (2008) The ratio of fibrinogen to red cells transfused affects survival in casualties receiving massive transfusions at an army combat support hospital. J Trauma 64:S79–S85
Fenger-Eriksen C, Lindberg-Larsen M, Christensen AQ, Ingerslev J, Sorensen B (2008) Fibrinogen concentrate substitution therapy in patients with massive haemorrhage and low plasma fibrinogen concentrations. Br J Anaesth 101:769–773
Weinkove R, Rangarajan S (2008) Fibrinogen concentrate for acquired hypofibrinogenaemic states. Transfus Med 18:151–157
Farriols Danés A, Gallur Cuenca L, Rodríguez Bueno S, Mendarte Barrenechea L, Bruno Montoro Ronsano J (2008) Efficacy and tolerability of human fibrinogen concentrate administration to patients with acquired fibrinogen deficiency and active or in high-risk severe bleeding. Vox Sang 94:221–226
Hiippala ST (1995) Dextran and hydroxyethyl starch interfere with fibrinogen assays. Blood Coagul Fibrinolysis 6:743–746
Fries D, Haas T, Klingler A, Streif W, Klima G, Martini J, Wagner-Berger H, Innerhofer P (2006) Efficacy of fibrinogen and prothrombin complex concentrate used to reverse dilutional coagulopathy—a porcine model. Br J Anaesth 97:460–467
Dickneite G, Pragst I (2009) Prothrombin complex concentrate vs fresh frozen plasma for reversal of dilutional coagulopathy in a porcine trauma model. Br J Anaesth 102:345–354
Schöchl H (2006) Coagulation management in major trauma. Hamostaseologie 26:S52–S55
Coats T, Roberts I, Shakur H (2004) Antifibrinolytic drugs for acute traumatic injury. Cochrane Database Syst Rev CD004896
Levy JH (2008) Pharmacologic methods to reduce perioperative bleeding. Transfusion 48:31S–38S
Franchini M (2007) The use of desmopressin as a hemostatic agent: a concise review. Am J Hematol 82:731–735
Carless PA, Henry DA, Moxey AJ, O’Connell D, McClelland B, Henderson KM, Sly K, Laupacis A, Fergusson D (2004) Desmopressin for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev CD001884
Zotz RB, Araba F, Bux I (2009) Desmopressin (DDAVP) for minimising perioperative allogenic blood transfusion: a stratified meta-analysis. Hamostaseologie 29:A53
Korte WC, Szadkowski C, Gahler A, Gabi K, Kownacki E, Eder M, Degiacomi P, Zoller N, Devay J, Lange J, Schnider T (2009) Factor XIII substitution in surgical cancer patients at high risk for intraoperative bleeding. Anesthesiology 110:239–245
Weeterings C, de Groot PG, Adelmeijer J, Lisman T (2008) The glycoprotein Ib–IX–V complex contributes to tissue factor-independent thrombin generation by recombinant factor VIIa on the activated platelet surface. Blood 112:3227–3233
Fries D, Haas T, Salchner V, Lindner K, Innerhofer P (2005) Management of coagulation after multiple trauma. Anaesthesist 54:137–144
Spinella PC, Perkins JG, McLaughlin DF, Niles SE, Grathwohl KW, Beekley AC, Salinas J, Mehta S, Wade CE, Holcomb JB (2008) The effect of recombinant activated factor VII on mortality in combat-related casualties with severe trauma and massive transfusion. J Trauma 64:286–293
Boffard KD, Riou B, Warren B, Choong PI, Rizoli S, Rossaint R, Axelsen M, Kluger Y (2005) Recombinant factor VIIa as adjunctive therapy for bleeding control in severely injured trauma patients: two parallel randomized, placebo-controlled, double-blind clinical trials. J Trauma 59:8–15
Stein DM, Dutton RP, Kramer ME, Scalea TM (2009) Reversal of coagulopathy in critically ill patients with traumatic brain injury: recombinant factor VIIa is more cost-effective than plasma. J Trauma 66:63–72
Stanworth SJ, Birchall J, Doree CJ, Hyde C (2007) Recombinant factor VIIa for the prevention and treatment of bleeding in patients without haemophilia. Cochrane Database Syst Rev CD005011
Duchesne JC, Mathew KA, Marr AB, Pinsky MR, Barbeau JM, McSwain NE (2008) Current evidence based guidelines for factor VIIa use in trauma: the good, the bad, and the ugly. Am Surg 74:1159–1165
Nishijima DK, Zehtabchi S (2009) The efficacy of recombinant activated factor VII in severe trauma. Ann Emerg Med 54:737–744
Spahn DR, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, Gordini G, Stahel PF, Hunt BJ, Komadina R, Neugebauer E, Ozier Y, Riddez L, Schultz A, Vincent JL, Rossaint R (2007) Management of bleeding following major trauma: a European guideline. Crit Care 11:R17
Dutton RP, Conti BM (2009) The role of recombinant-activated factor VII in bleeding trauma patients. Curr Opin Anaesthesiol 22:299–304
Mannucci PM, Levi M (2007) Prevention and treatment of major blood loss. N Engl J Med 356:2301–2311
Hsia CC, Chin-Yee IH, McAlister VC (2008) Use of recombinant activated factor VII in patients without hemophilia: a meta-analysis of randomized control trials. Ann Surg 248:61–68
Afshari A, Wetterslev J, Brok J, Moller A (2007) Antithrombin III in critically ill patients: systematic review with meta-analysis and trial sequential analysis. BMJ 335:1248–1251
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lier, H., Böttiger, B.W., Hinkelbein, J. et al. Coagulation management in multiple trauma: a systematic review. Intensive Care Med 37, 572–582 (2011). https://doi.org/10.1007/s00134-011-2139-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-011-2139-y