Abstract
Objective
Despite an overall correlation between the bispectral index of the EEG (BIS) and clinical sedation assessment, unexpectedly high BIS values can be observed at deep sedation levels. We assessed the frequency, interindividual variability and clinical impact of high BIS values during clinically deep sedation.
Design and setting
Prospective observational study in two university-affiliated intensive care units.
Patients
Sixty-two mechanically ventilated patients requiring intravenous sedation and analgesia for ≥24 h.
Measurements and main results
Paired measurements of BIS and sedation measured on the adaptation to intensive care environment (ATICE) score were obtained every 3 h until awakening. A paired measurement with BIS >60 at deep sedation (ATICE Awakeness ≤2) was defined as discordant. Patients were considered discordant if their individual ratio of number of discordant measurements to number of total measurements during deep sedation was above the median discordance ratio of the overall cohort. At least one discordant assessment was observed in 52 patients (83.9%). Median individual discordance ratio was 32% (14.3–50.0%). Time from awakening to first T-piece trial [16 h (4–34) vs. 46 h (9–109), p = 0.01] and to extubation [35 h (23–89) vs. 88 h (46–152 h), p = 0.05] were significantly shorter in discordant compared to concordant patients. BIS-ATICE discordance was independently associated with successful extubation within 48 h after awakening (OR 6.7, CI 95% 1.8–25.0, p = 0.005). The rate of ICU recall was not different in BIS-ATICE discordant and concordant patients.
Conclusions
In mechanically ventilated ICU patients, discordance between high BIS values and deep clinical sedation is frequently observed and may suggest faster weaning from the ventilator.
Similar content being viewed by others
Introduction
Intravenous sedation and analgesia are cornerstones in the management of mechanically ventilated patients in the intensive care unit (ICU) [1]. Clinical scales have been recently developed and validated for monitoring sedation and analgesia in these patients [2–6], enabling the avoidance of both under and over sedation. Although correlations between the bispectral index of the EEG (BIS) and the clinical sedation scale have been reported [7–9], large ranges of BIS values at various predefined sedation levels are commonly observed in ICU patients, whatever BIS version is used [10–13]. In particular, unexpectedly high BIS values can be observed at clinical deep sedation levels when low BIS values would be expected. Regardless of whether the unexpectedly high BIS signal is of muscular or cerebral origin [14], the frequency of such findings remains to be determined. Furthermore, it has not been established whether patients demonstrating frequently or infrequently high BIS values during deep sedation are different in respect of risk factors and ICU outcome. Here, we hypothesized that unexpectedly high BIS values during deep sedation could indicate a preserved cerebral or muscular activity, which may unmask after sedation withdrawal and awakening and impact on duration of mechanical ventilation or rate of ICU recalls.
The main goal of the study was to assess the frequency and inter-individual variability of high BIS values during episodes of deep sedation. We expected a large interindividual variability in discordance between BIS and clinical sedation level. Secondary objectives were to compare patients with frequently and infrequently high BIS values during deep sedation in respect of duration of weaning from mechanical ventilation and incidence of ICU recall. We also sought to compare these patients in terms of demographic factors and factors related to the acute condition and treatment.
Materials and methods
Patients
The protocol was approved by the Ethics Committee of Saint-Germain en Laye Hospital (Saint-Germain, France). We conducted a prospective, bicenter study in two university-affiliated ICUs. All patients requiring continuous intravenous sedation and analgesia for mechanical ventilation scheduled for more than 24 h were eligible. Exclusion criteria were presence of neurological or neuromuscular disorders (except isolated traumatic paraplegia), admission for cardiac arrest, admission from another ICU and unavailability of BIS monitor. The study was conducted over an 18-month period of time.
BIS recordings and clinical assessment of sedation
BIS values were recorded using the BIS®–XP 2000 monitor (software version 3.12) developed by Aspect Medical System™, according to the manufacturer's recommendations (see Electronic supplementary materials). Immediately after each BIS measurement, the level of sedation was assessed using the Awakeness domain of the ATICE scale (ATICE Awakeness). The score ranged from 0 (patient areactive to noxious stimulus) to 5 (eyes open spontaneously) (Table 1). The ATICE score has been shown accurate in the ICU setting, including validity, reproducibility and responsiveness [5]. Nurses in both centers were familiar with the use of the ATICE scale and BIS. The BIS value and ATICE Awakeness score were recorded simultaneously every 3 h well after patient positioning, endotracheal suction or any painful stimulus. BIS and ATICE Awakeness scores were recorded from onset time of intravenous sedation until awakening. Awakening was defined by an ATICE Awakeness score of 5 on two consecutive assessments during or after a progressive decrease in sedatives and analgesics. BIS value and ATICE scores were not recorded during the use of neuromuscular blockers (cisatracurium in both centers). The protocol used to monitor neuromuscular blockade is available as Electronic Supplementary Material.
Management of sedation and analgesia
Midazolam and fentanyl were used as hypnotic and analgesic drugs, respectively. Dosages were adjusted following a previously published algorithm [15], available in the Electronic Supplementary Materials. Briefly, the aim of the algorithm was to promote light sedation once tolerance to the ICU environment, including comfort, analgesia, calmness and adaptation to the ventilator, was achieved, which could transiently require, especially in those patients with severe respiratory failure, high dosages of sedatives and analgesics, resulting in altered consciousness. This strategy is in accordance to the French Society of Critical Care 2007 guidelines [16]. Daily interruption of sedatives and analgesics or analgesia-based sedation were not performed. Propofol or remifentanil could be used based on physician’s decision in patients with poor tolerance to the ICU environment despite high doses of midazolam or fentanyl, or to ensure tolerance within the few hours prior to extubation.
Weaning from mechanical ventilation
Weaning from mechanical ventilation was conducted according to the French ICU Consensus Conference 2003 guidelines [17]. After awakening (as defined above), patients were screened daily for the presence of prerequisite criteria for extubation, including FiO2 <50%, PEEP <5 cm, no high-grade fever and no catecholamine infusion (except low doses of dopamine or dobutamine). From the day prerequisite criteria were fulfilled, patients underwent daily T-piece trials of 30–120-min duration. Satisfactory tolerance of the T-piece trial assessed using simple bedside tolerance parameters including respiratory rate, SpO2 and the use of accessory respiratory muscles was followed by extubation. Weaning was considered successful when re-ventilation was not required within the next 48 h.
Neuromuscular testing and screening for ICU recall
Limb muscle strength was assessed within the 48 h following awakening using the Medical Research Council (MRC) score [18]. Patients were screened for delirium within 48 h following ICU discharge. Delirium was defined as a intensive care delirium screening checklist score ≥4 [19, 20]. Patients without delirium were then interviewed using the ICU stressful experiences questionnaire [21] and the ICU memory tool [22] (see Electronic Supplementary Material).
Statistical analysis
Results are expressed as number and percentage, or median and interquartile (IQR). All statistical analyses were conducted using JMP™ software, version 7 (SAS Institute Inc., Cary, NC). Compliance with the study protocol was determined by the ratio of the number of actual BIS-ATICE paired measurements obtained to the theoretical total number of expected paired measurements. Patients with a compliance ratio <50% were excluded from analysis. Overall correlation between BIS and ATICE Awakeness was assessed using the Spearman coefficient. To avoid bias introduced by a high number of repeated assessments of an individual patient, we also measured the Spearman coefficient using only three random sets of paired measurements per patient.
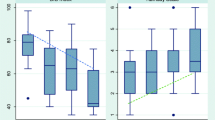
As our study represents the first attempt to quantify the rate of unexpectedly high BIS values during deep sedation, we chose robust and clinically relevant criteria to categorize patients. Deep sedation was defined as an ATICE Awakeness score of 0, 1 or 2 (no response to any stimulation or response to a strong stimulation only), corresponding to a level 6 on the Ramsay scale. Since previous studies showed that in deeply sedated patients BIS values were equally distributed around a value close to 60 [7, 9, 11, 23], we used this threshold a priori to distinguish concordant sedation assessments (deep sedation, i.e., ATICE Awakeness 0, 1 or 2, with concomitant BIS ≤60) and discordant assessments (deep sedation with concomitant BIS >60).
For each patient, a discordance ratio was defined as the ratio of the number of discordant assessments to the number of total assessments obtained during deep sedation (i.e., with an ATICE Awakeness score of 0, 1 or 2). As no commonly accepted threshold exists to distinguish frequent and infrequent recordings of high BIS values during deep sedation, we defined a priori a patient as discordant (concordant) when the individual discordance ratio was greater (lesser than or equal to, respectively) to the median discordance ratio of the overall cohort. Discordant and concordant patients were compared in respect of demographic, clinical and treatment data using the chi-square test and Fischer’s exact test as appropriate for categorical variables, and the Mann–Whitney U test for quantitative variables. A p value ≤ 0.05 was considered significant.
BIS-ATICE discordance and other relevant variables were compared in patients with a time from awakening to extubation ≤ and >48 h in univariate analysis. Impact of BIS-ATICE discordance on extubation rate within 48 h after awakening was adjusted in a logistic regression analysis for the two variables with the lowest p value in univariate analysis regardless of a p value threshold.
Results
Seventy-five patients were included, 13 of which were then excluded from the analysis because of poor compliance with the study protocol due to transient high nurse workload (n = 5), intracerebral hemorrhage (n = 1) and post-anoxic coma (n = 1) after inclusion, repeated BIS sensor malfunction (n = 3) and deep sedation (ATICE scores of 0, 1 or 2) <24 h (n = 3). A total of 62 patients were analyzed. Baseline characteristics are presented in Table 2. Time from onset of sedation to awakening (as defined in “Materials and Methods”) was 119 h (59–188). Compliance ratio of BIS-ATICE measurements was 82% (74–93). Of the measurements, 59.5% (40.5–79.2) proved deep sedation (ATICE Awakeness 0, 1 or 2).
The number of paired BIS-ATICE assessments was 2,101, representing a mean of 33.8 assessments per patient from sedation onset to awakening. Median BIS value was 59, ranging from 4 to 98, with 25th and 75th percentiles of 43 and 79, respectively. BIS values against ATICE score values are displayed in a plot diagram in Fig. 1. The Spearman coefficient for the overall correlation between BIS and ATICE using all paired BIS-ATICE measurements was 0.43 (p < 0.001). When only three random sets of paired measurements per patient were used, the Spearman coefficient was 0.35 (p < 0.001). At least one discordant assessment (BIS value >60 with an ATICE Awakeness of 0, 1 or 2) was observed in 52 patients (83.9%). Seven patients (11.3%) showed all assessments discordant. The median individual ratio of discordance was 32.4% (14.3–50.0%). This value was used to separate discordant and concordant patients. Median BIS value during episodes of deep sedation was 61 (44–77) in the discordant patients and 44 (38–56) in the concordant patients (p < 0.0001).
Comparison of characteristics between patients with individual discordance ratio less or equal to (concordant patients) and greater to (discordant patients) 32.4% on admission and from sedation onset to awakening showed no significant difference in terms of age, severity or type of illness, chronic alcohol abuse, painful condition and sepsis (Table 2). Similarly, time from onset of sedation to awakening (as defined in “Materials and Methods”) and dosage of sedatives and opioids delivered were not significantly different.
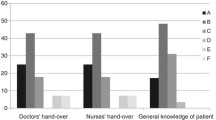
Time from awakening to first T-piece trial was significantly shorter in discordant patients [16 h (4–34)] compared to concordant patients [46 h (9–109), p = 0.01, Fig. 2]. Similarly, time from awakening to extubation was significantly shorter in discordant patients [35 h (23–89) vs. 88 h (46–152), p = 0.05, Fig. 2]. Successful extubation within 48 h after awakening was significantly more frequent in discordant patients [18 (69.2%) vs. 8 (30.8%), p = 0.006, RR = 2.3 (95% CI 1.2–4.2), Table 3]. After adjustment for admission SAPS II and time from MV onset to awakening (the two variables with the lowest p value in univariate analysis apart from BIS-ATICE discordance), BIS-ATICE discordance remained independently associated with a successful extubation within 48 h after awakening (odds ratio 6.7, 95% CI 1.8–25.0, p = 0.005).
Comparisons of MV duration prior to awakening, and time from awakening to 1st T-piece trial and to extubation between discordant (light gray bars) and concordant (dark gray bars) patients. Data are expressed as median and interquartile ranges. Discordant and concordant patients were defined by a discordance ratio (ratio of the number of measurements with a BIS value >60 during deep sedation to the number of total measurements obtained during deep sedation) > and ≤32%, respectively. Awakening was defined by an ATICE Awakeness score of 5 on 2 consecutive assessments
MRC score measured within 48 h after extubation was not different between discordant [49 (42–54)] and concordant [48 (38–54)] patients (p = 0.6). Forty-nine patients were discharged alive from the ICU [25 (80.6%) discordant and 24 (77.4%) concordant patients, p = 0.8]. Sixteen of them were not assessed for ICU recalls for the following reasons: intensive care delirium screening checklist delirium score ≥4 (n = 4), transfer to another hospital (n = 1), language barrier (n = 1), mental backwardness (n = 1) and miscellaneous reasons (n = 9). Accordingly, 33 patients (17 discordant and 16 concordant patients, p = 0.8) were assessed for ICU recalls within 48 h following ICU discharge (Table 4). The adapted ICU stressful experiences questionnaire score was not statistically different between concordant [51 (35–67)] and discordant patients [45 (36–61), p = 0.4]. Using the ICU memory tool, all patients remembered their ICU stay. Six discordant patients and seven concordant patients remembered lucidly their stay in the ICU (p = 0.7). There was no significant difference between discordant and concordant patients in terms of recall of factual events, feelings or delusions (Table 4).
Discussion
The overall correlation between BIS and clinically assessed sedation in our study, whether patients were or not deeply sedated, was only moderately significant, with an important overlap in BIS values across ATICE Awakeness categories. Although such a moderate correlation has already been reported [7], other studies have demonstrated a higher (though still moderate) correlation [9, 11]. Comparing correlation coefficients across studies and populations is complex due to heterogeneity of populations, different methods of collecting data and different BIS software and hardware [24]. For example, in our study, contrary to others, paired measurements of BIS and clinical sedation status were performed all over the sedation period, leading to a high number of paired assessments per patients (an average of 30). This represents a non-selected real-life comparison of BIS versus clinical sedation level. During deep sedation, unexpectedly high BIS values were particularly frequent. Over 80% of the patients did show at least one episode of discordance. The median individual discordance rate of 32% indicates that 50% of patients had at least one-third of their BIS values above 60 during deep sedation. This finding does not support the use of BIS as a surrogate of clinical sedation assessment for adapting dosage of sedation in mechanically ventilated patients.
Although unexpectedly high BIS signal has been attributed to a sustained face (or scalp) muscle EMG signal despite deep sedation [14], we did not aim to reproduce this finding. Our study represents rather more an attempt to describe clinical factors associated with the occurrence of high BIS during clinically deep sedation, regardless of whether unexpectedly high BIS values were due to a preserved cerebral or muscle signal or both. Acute pain is a cause of a rise in BIS value in ICU patients [25]. Similarly, patients with chronic alcohol consumption are at high risk of in-hospital withdrawal syndrome [26] and may therefore demonstrate preserved cortical activity with apparent clinical deep sedation. Finally, BIS values are generally affected by intravenous sedatives (propofol, midazolam), while opioids show little impact on them [27, 28]. However, in our study neither presence of a painful condition, nor chronic alcoholism, nor the nature or amount of sedatives or analgesics administered were associated with BIS-ATICE discordance. This suggests that discordance between high BIS values and clinical deep sedation levels might depend primarily on individual intrinsic factors.
We found that discordant patients, i.e., those with preserved cerebral or face muscle activity during deep sedation, had a shorter mechanical ventilation weaning process. The time to extubation was halved and rate of successful extubation within 48 h after awakening over doubled compared to concordant patients. The favorable impact on the weaning process remained after adjustment for the two variables with the lowest p value in univariate analysis (admission SAPS II and duration of MV prior to awakening). Face muscle origin BIS-recorded electrical activity could have been preserved in patients who did not develop critical illness neuromyopathy, a common cause for delayed weaning [29]. However, muscle strength measured at awakening in the four limbs was not significantly different between discordant and concordant patients. More subtle differences in strength and tone in regional muscles, including laryngeal and swallowing muscles, both involved in the upper airway protection after extubation, cannot be excluded. Most of the gain in time to extubation in discordant patients was observed during the time from awakening to that of the first T-piece trial. This suggests that the first T-piece trial could be initiated earlier in patients who previously exhibited frequent discordances between high BIS values and deep clinical sedation levels. After awakening, these patients might have regained a satisfactory level of interaction with caregivers faster than concordant patients. This could have unconsciously prompted physicians to initiate the first T-piece trial earlier.
All patients who answered the post-ICU questionnaires remembered undergoing mechanical ventilation and ICU stay. Recall of stressful experiences was frequent. Such findings are common among critically ill patients who have been mechanically ventilated [21, 30, 31]. However, the number of stressful experiences reported and their intensity were not significantly different between discordant and concordant patients. ICU patients mainly remember events occurring at the end of their stay in the ICU [32], when sedation has been lightened. Our results suggest that a high BIS value during sedation does not increase the ability to remember events after awakening.
Our study has several limitations. Nurses were not blind to the record of the BIS value before measuring the ATICE Awakeness score. However, nurses were not aware of the BIS threshold of 60 subsequently used to separate BIS-ATICE paired assessments. Furthermore, the high intra-class correlation coefficient of 0.99 for the consciousness domain of the ATICE [5] reflects that this measurement is minimally affected by subjectivity. Although we used the median value of the individual ratio of high BIS values to the total number of paired BIS-ATICE measurements during deep sedation to define discordant and concordant patients, the exact rate of high BIS values during deep sedation above which patients should be considered discordant remains to be established. The number of patients assessed at ICU discharge was relatively small, which might have precluded the recognition of significant differences in post-ICU recall between discordant and concordant patients. Finally, the follow-up ended at ICU discharge, and the impact of BIS-ATICE discordance on the medium- or long-term was not assessed.
Conclusions
Although light sedation is increasingly recognized as feasible, safe and beneficial in the critically ill [15, 33–36], some patients still may require transiently high doses of sedatives and analgesics to provide strict adaptation to the ventilator. This often results in deep alteration of consciousness. Our study results suggest that BIS may contribute to distinguishing two different patterns in patient responses to deep sedation; some patients maintain high BIS levels during deep sedation and others not. Those maintaining high BIS values may demonstrate faster weaning from the ventilator. Whether BIS response to deep sedation is a true reflection of individual patient characteristics or can be modified by therapeutic interventions warrants further investigation. If BIS response to deep sedation can be altered by modifying sedatives and analgesics dosages or other interventions, then trying to maintain a high BIS value when deep sedation is unavoidable may represent a therapeutic goal. If BIS response to deep sedation reflects patient’s individual characteristics, then BIS may be useful to assess comparability in groups of patients receiving sedatives. As a consequence, BIS might prove to be complementary to clinical sedation assessment during periods of deep sedation.
References
Payen JF, Chanques G, Mantz J, Hercule C, Auriant I, Leguillou JL, Binhas M, Genty C, Rolland C, Bosson JL (2007) Current practices in sedation and analgesia for mechanically ventilated critically ill patients: a prospective multicenter patient-based study. Anesthesiology 106:687–695
De Jonghe B, Cook D, Appere-De-Vecchi C, Guyatt G, Meade M, Outin H (2000) Using and understanding sedation scoring systems: a systematic review. Intensive Care Med 26:275–285
Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, Francis J, Speroff T, Gautam S, Margolin R, Sessler CN, Dittus RS, Bernard GR (2003) Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond agitation–sedation scale (RASS). JAMA 289:2983–2991
Riker RR, Picard JT, Fraser GL (1999) Prospective evaluation of the sedation–agitation scale for adult critically ill patients. Crit Care Med 27:1325–1329
De Jonghe B, Cook D, Griffith L, Appere-de-Vecchi C, Guyatt G, Théron V, Vagnère A, Outin H (2003) Adaptation to the intensive care environment (ATICE): development and validation of a new sedation assessment instrument. Critical Care Med 31:2344–2354
Payen JF, Bru O, Bosson JL, Lagrasta A, Novel E, Deschaux I, Lavagne P, Jacquot C (2001) Assessing pain in critically ill sedated patients by using a behavioral pain scale. Crit Care Med 29:2258–2263
Simmons LE, Riker RR, Prato BS, Fraser GL (1999) Assessing sedation during intensive care unit mechanical ventilation with the bispectral index and the sedation–agitation scale. Crit Care Med 27:1499–1504
Riker RR, Fraser GL, Simmons LE, Wilkins ML (2001) Validating the sedation–agitation scale with the bispectral index and visual analog scale in adult ICU patients after cardiac surgery. Intensive Care Med 27:853–858
Ely EW, Truman B, Manzi DJ, Sigl JC, Shintani A, Bernard GR (2004) Consciousness monitoring in ventilated patients: bispectral EEG monitors arousal not delirium. Intensive Care Med 30:1537–1543
Frenzel D, Greim CA, Sommer C, Bauerle K, Roewer N (2002) Is the bispectral index appropriate for monitoring the sedation level of mechanically ventilated surgical ICU patients? Intensive Care Med 28:178–183
Nasraway SS Jr, Wu EC, Kelleher RM, Yasuda CM, Donnelly AM (2002) How reliable is the bispectral index in critically ill patients? A prospective, comparative, single-blinded observer study. Crit Care Med 30:1483–1487
Courtman SP, Wardurgh A, Petros AJ (2003) Comparison of the bispectral index monitor with the comfort score in assessing level of sedation of critically ill children. Intensive Care Med 29:2239–2246
Tonner PH, Paris A, Scholz J (2006) Monitoring consciousness in intensive care medicine. Best Pract Res Clin Anaesthesiol 20:191–200
Vivien B, Di Maria S, Ouattara A, Langeron O, Coriat P, Riou B (2003) Overestimation of bispectral index in sedated intensive care unit patients revealed by administration of muscle relaxant. Anesthesiology 99:9–17
De Jonghe B, Bastuji-Garin S, Fangio P, Lacherade JC, Jabot J, Appere-De-Vecchi C, Rocha N, Outin H (2005) Sedation algorithm in critically ill patients without acute brain injury. Crit Care Med 33:120–127
(2007) 4ème conférence de consensus commune: sédation et analgésie en réanimation (nouveau-né exclus). http://www.srlf.org/Data/ModuleGestionDeContenu/application/714.pdf
Richard C, Beydon L, Cantagrel S, Cuvelier A, Fauroux B, Garo B (2001) Sevrage de la ventilation mécanique (à l’exclusion du nouveau-né et du réveil d’anesthésie). http://www.srlf.org/Data/ModuleGestionDeContenu/PagesGenerees/Bibliothèque%20-%20Référentiels/Référentiels/Recommandations/CC/139.asp
Kleyweg RP, van der Meche FG, Meulstee J (1988) Treatment of Guillain–Barre syndrome with high-dose gammaglobulin. Neurology 38:1639–1641
Dubois MJ, Bergeron N, Dumont M, Dial S, Skrobik Y (2001) Delirium in an intensive care unit: a study of risk factors. Intensive Care Med 27:1297–1304
Devlin JW, Fong JJ, Schumaker G, O’Connor H, Ruthazer R, Garpestad E (2007) Use of a validated delirium assessment tool improves the ability of physicians to identify delirium in medical intensive care unit patients. Crit Care Med 35:2721–2724
Rotondi AJ, Chelluri L, Sirio C, Mendelsohn A, Schulz R, Belle S, Im K, Donahoe M, Pinsky MR (2002) Patients’ recollections of stressful experiences while receiving prolonged mechanical ventilation in an intensive care unit. Crit Care Med 30:746–752
Jones C, Griffiths RD, Humphris G, Skirrow PM (2001) Memory, delusions, and the development of acute posttraumatic stress disorder-related symptoms after intensive care. Crit Care Med 29:573–580
Mondello E, Siliotti R, Noto G, Cuzzocrea E, Scollo G, Trimarchi G, Venuti FS (2002) Bispectral index in ICU: correlation with Ramsay Score on assessment of sedation level. J Clin Monit Comput 17:271–277
LeBlanc JM, Dasta JF, Kane-Gill SL (2006) Role of the bispectral index in sedation monitoring in the ICU. Ann Pharmacother 40:490–500
Brocas E, Dupont H, Paugam-Burtz C, Servin F, Mantz J, Desmonts JM (2002) Bispectral index variations during tracheal suction in mechanically ventilated critically ill patients: effect of an alfentanil bolus. Intensive Care Med 28:211–213
Spies CD, Rommelspacher H (1999) Alcohol withdrawal in the surgical patient: prevention and treatment. Anesth Analg 88:946–954
Struys MM, Vereecke H, Moerman A, Jensen EW, Verhaeghen D, De Neve N, Dumortier FJ, Mortier EP (2003) Ability of the bispectral index, autoregressive modelling with exogenous input-derived auditory evoked potentials, and predicted propofol concentrations to measure patient responsiveness during anesthesia with propofol and remifentanil. Anesthesiology 99:802–812
Struys MM, De Smet T, Greenwald S, Absalom AR, Binge S, Mortier EP (2004) Performance evaluation of two published closed-loop control systems using bispectral index monitoring: a simulation study. Anesthesiology 100:640–647
De Jonghe B, Bastuji-Garin S, Sharshar T, Outin H, Brochard L (2004) Does ICU-acquired paresis lengthen weaning from mechanical ventilation? Intensive Care Med 30:1117–1121
van de Leur JP, van der Schans CP, Loef BG, Deelman BG, Geertzen JH, Zwaveling JH (2004) Discomfort and factual recollection in intensive care unit patients. Crit Care 8:R467–R473
Rundshagen I, Schnabel K, Wegner C, am Esch S (2002) Incidence of recall, nightmares, and hallucinations during analgosedation in intensive care. Intensive Care Med 28:38–43
Weinert CR, Sprenkle M (2008) Post-ICU consequences of patient wakefulness and sedative exposure during mechanical ventilation. Intensive Care Med 34:82–90
Kress JP, Vinayak AG, Levitt J, Schweickert WD, Gehlbach BK, Zimmerman F, Pohlman AS, Hall JB (2007) Daily sedative interruption in mechanically ventilated patients at risk for coronary artery disease. Crit Care Med 35:365–371
Brook AD, Ahrens TS, Schaiff R, Prentice D, Sherman G, Shannon W, Kollef MH (1999) Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med 27:2609–2615
Brattebo G, Hofoss D, Flaatten H, Muri AK, Gjerde S, Plsek PE (2002) Effect of a scoring system and protocol for sedation on duration of patients’ need for ventilator support in a surgical intensive care unit. Br Med J 324:1386–1389
Quenot JP, Ladoire S, Devoucoux F, Doise JM, Cailliod R, Cunin N, Aube H, Blettery B, Charles PE (2007) Effect of a nurse-implemented sedation protocol on the incidence of ventilator-associated pneumonia. Crit Care Med 35:2031–2036
Le Gall JR, Lemeshow S, Saulnier F (1993) A new simplified acute physiologic score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
Acknowledgments
Only institutional funding supported this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Trouiller, P., Fangio, P., Paugam-Burtz, C. et al. Frequency and clinical impact of preserved bispectral index activity during deep sedation in mechanically ventilated ICU patients. Intensive Care Med 35, 2096–2104 (2009). https://doi.org/10.1007/s00134-009-1636-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-009-1636-8