Abstract
Objective
To evaluate the ability of ∆PP/∆P [pulse pressure variations (∆PP) adjusted by alveolar pressure variations (∆P = Pplat-PEEPtot)] in predicting fluid responsiveness, to compare its accuracy to that of ∆PP used alone and to evaluate the influence of tidal volume (Vt) on these two indices.
Design
Prospective study.
Setting
A 22-bed general intensive care unit (ICU).
Patients
Eighty-four surgical or medical ventilated patients requiring fluid challenge.
Intervention
A 6 ml/kg colloid fluid challenge in 30 min.
Measurements and results
Hemodynamic measurements taken before and after fluid challenge. Patients separated into responders and nonresponders according to a 15% increase in their cardiac output. Thirty-nine patients found to be responders and 45 nonresponders. ∆PP/∆P and ∆PP were both higher in responders than in nonresponders. ∆PP/∆P was a better predictor of fluid responsiveness than ∆PP, especially for patients ventilated with Vt ≥ 8 ml/kg [area under the curve (AUC) 0.88 (0.77–0.98) versus 0.75 (0.60–0.89), P < 0.01)]. In this population ∆PP/∆P higher than 0.9 predicted fluid response with positive predictive value of 87% and negative predictive value of 78%. Overall ∆PP and ∆PP/∆P reliability was poor for patients ventilated with Vt < 8 ml/kg [AUC 0.63 (0.45–0.81) and 0.72 (0.55–0.88), respectively].
Conclusion
In this mixed ICU population ∆PP adjusted by ∆P is a simple index which outperforms ∆PP for patients ventilated with Vt ≥ 8 ml/kg. However, correcting ∆PP by ∆P still fails to predict fluid response reliably in patients ventilated with low tidal volume.
Similar content being viewed by others
Introduction
Adequate use of fluid therapy in critically ill patients is a major issue for intensive care physicians. As Starling curve changes between patients, static markers of preload poorly reflect the prediction of fluid responsiveness [1, 2]. In contrast, dynamic indices related to cyclic change in left ventricular stroke volume induced by mechanical ventilation usually predict individual response to fluid loading [3–6]. Michard et al. [6] have demonstrated that respiratory changes in stroke volume can be evaluated reliably by changes in pulse pressure (∆PP). However, several authors note that the formation of ∆PP is complex and depends on the volume and respiratory status of the patient [7, 8]. For a preload-dependent patient the main stimulus of pulse pressure variation is likely to be the inspiratory decrease in right ventricular preload due to the increase in pleural pressure [7]. Pleural and intrathoracic pressure variations are therefore the motor of cyclic change in stroke volume in preload-dependent patients. This depends on alveolar pressure variations and lung compliance influencing the transmission of alveolar pressure to the pleural space (transpulmonary pressure) [8]. This physiological point may explain, at least in part, the limitations of ∆PP recently underlined in patients ventilated with low tidal volume [9] and/or patients with right ventricular dysfunction [10].
We hypothesized that normalizing the pulse pressure variation (∆PP) by alveolar pressure variations (∆P = Pplat-PEEPtot) (Fig. 1 of ESM) could give a combined index (∆PP/∆P) which could attenuate some of the false-negative and false-positive results of ∆PP. False negative occurs when the changes in transpulmonary pressure are too small to induce changes in venous return: small ∆PP could be normalized by a small ∆P. The false positive occurs when the presence of a significant ∆PP may be the consequence of a right ventricular (RV) dysfunction: inordinate increase of RV output impedance with an enlargement of ∆P unbalanced by substantial decrease of venous return.
The aim of this study was thus to compare ∆PP/∆P and ∆PP as indicators of fluid responsiveness in a mixed population of ICU patients. The second aim was to evaluate the influence of tidal volume on these two indices.
Materials and methods
Methods
From July 2005 to July 2007 all patients ventilated and monitored by PiCCo system and for whom a fluid challenge was discussed according to the clinical presentation were included in the present study. In each patient an indication of fluid loading was given on the basis of tachycardia, hypotension, oliguria or cutaneous vasoconstriction. All patients were monitored with arterial catheter as well as by cardiac monitoring by PiCCo system (Pulsion, Medical Systems AG, Munich, Germany). Patients were mechanically ventilated without spontaneous respiratory movements (identified by visual inspection of the airway pressure curve and leaking of assisted breath when compared with the respiratory rate set on the ventilator). Patients were excluded in case of arrhythmia. Fluid challenge was performed with 6 ml/kg hydroxyethyl starch solution (Voluven, Fresenius KABI®, Germany) infused over 30 min. Patients were considered fluid responders when cardiac index (CI) increased over more than 15% of baseline value. Hemodynamic measurements obtained just before and after the fluid challenge included mean arterial pressure (MAP), heart rate (HR), central venous pressure (CVP), and CI. CI was determined by the average of three thermodilution measurements using 15 ml cold physiologic serum. ∆PP was determined as previously described using the maximal pulse pressure at end-inspiration and the minimal pulse pressure obtained 3–4 beats later during expiration by manual measurement of arterial pressure from a conventional arterial catheter after performing a fast-flush test [11]. Recordings were made directly on scope (Philips®V50) using cursors after freezing windows.
Respiratory measurements obtained before fluid challenge included expiratory tidal volume (Vt), respiratory rate (RR), total PEEP (PEEPtot), and plateau pressure (Pplat). PEEPtot and Pplat were measured using end-expiratory and end-inspiratory occlusion maneuvers of 2 s, respectively. ∆PP/∆P was obtained by dividing ∆PP by (Pplat-PEEP) (Fig. 1 of ESM). Static compliance (Cs) was calculated by dividing Vt by (Pplat-PEEPtot). The ratio of ∆PP/Vt to Vt expressed in ml/kg was also tested to assess the impact of Vt level on ∆PP value. Patients were ventilated by the following ventilators at the discretion of the treating physician: Evita 4 (Drager®); Servo300 or Servo I (Siemens®). Ideal body weight was calculated using the following ideal weight formula: Ideal weight = X + 0.9 × (Height (cm) − 152); ♀: X = 45.5 kg, ♂: X = 50 kg.
The study was approved by the Ethics Committee of our institution. Consent was waived as no specific intervention was required. The fluid loading procedure and the hemodynamic measurements taken in the present study are routinely performed to assess fluid responsiveness in our unit.
Statistical analysis
Parameters were compared using analysis of variance (ANOVA) or nonparametric Mann–Whitney U test when equality of variance was not met. The effects of fluid challenge were analyzed using Wilcoxon rank test. Receiver operator characteristic (ROC) curves were first generated for all patients to evaluate the capacity of ∆PP and ∆PP/∆P to predict fluid responsiveness. If the ROC curve area was greater than 0.5 the best cutoff value was calculated. Then the ROC curve analysis was generated, separating patients according to tidal volume of <8 ml/kg and ≥8 ml/kg. The same analysis was done for the ∆PP/Vt ratio. P value ≤0.05 was considered statistically significant. Data are presented as median (25–75 percentiles) and with 95% confidence interval for the predictive value and ROC curve area.
Results
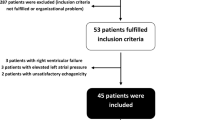
From July 2005 to July 2007, 84 patients were successively enrolled. Based on CI variation induced by the fluid challenge, 39 patients were classified as responders and 45 as nonresponders. There was no significant difference between responders and nonresponders according to age, simplified acute physiology score II (SAPSII), diagnosis, and norepinephrine infusion rate (Table 1).
In the overall population ∆PP and ∆PP/∆P was higher in responders than in nonresponders (Table 2). The prediction of fluid responsiveness was better with ∆PP/∆P than ∆PP [ROC curve area (AUC) 0.81 (0.71–0.90) versus 0.71 (0.60–0.82); P < 0.01] (Table 4).
At baseline, CI was lower in responders than in nonresponders (Table 2): AUC for CI baseline value was 0.63 (0.45–0.79).
Tidal volume was <8 ml/kg in 42 patients and ≥8 ml/kg in 42 patients. For Vt ≥ 8 ml/kg, ∆PP/∆P was a significantly better predictor of fluid responsiveness than ∆PP [ROC curve area 0.88 (0.77–0.98) versus 0.75 (0.60–0.89); P < 0.01] (Figs. 1 and 2; Table 4). Characteristics of patients ventilated with tidal volume <8 ml/kg and ≥8 ml/kg are presented in Table 3. For Vt < 8 ml/kg, ∆PP and ∆PP/∆P failed to predict fluid responsiveness accurately [ROC curve area 0.62 (0.45–0.80) and 0.72 (0.55–0.88)] (Table 4).
ROC curves comparing the ability of ∆PP/∆P and ∆PP to distinguish responder and nonresponder patients to fluid challenge for patients ventilated with tidal volume ≥8 ml/kg [ROC curve area 0.88 (0.77–0.98) for ∆PP/∆P versus 0.75 (0.60–0.89) for ∆PP; P < 0.01]. The best cutoff values were 0.9 for ∆PP/∆P and 15% for ∆PP
In the overall population, as well as in patients ventilated with high or low Vt, the ∆PP/Vt ratio did not perform better than ∆PP (Fig. 2 of ESM).
Discussion
Our results obtained from a group of unselected ICU patients show first that ∆PP adjusted by ∆P is a simple index which outperforms ∆PP for patients ventilated with Vt ≥8 ml/kg. Secondly this study confirmed that ∆PP reliability was poor for patients ventilated with Vt <8 ml/kg, and reveals that ∆PP/∆P failed to really improve overall prediction at low Vt.
Pulse pressure variations depend on both cardiovascular and respiratory mechanisms [12, 13]. On the respiratory side these processes include at least: Vt level, lung volume, PEEP level, and chest wall and lung compliances [14–16]. This emphasizes that ∆PP should not be interpreted without paying attention to ventilatory settings [17, 18]. With this objective the simple normalization—easily obtained at the bedside—of respiratory pulse pressure variations by alveolar pressure variations has increased the accuracy of ∆PP by diminishing the number of false negatives and false positives, especially in patients ventilated with Vt ≥8 ml/kg (Figs. 1 and 2). As mentioned previously two explanations may be advanced to support our results.
The first scenario occurs in the case of low changes in transpulmonary pressure while preload reserve is present. In this case, an increase in lung volume and airway pressure may not be large enough to modify pleural pressure, venous return, and cardiac filling [17]: A relatively inconsistent ∆PP may be counterbalanced by a low ∆P, and a combined index (∆PP/∆P) may therefore be more likely to reveal underestimated volume responsiveness. Romand et al. demonstrated that cyclic change in pleural pressure is mostly determined by magnitude of tidal volume (Vt) but also to a lesser extent by driving pressure variations (∆P) [19]. In fact, few studies have argued that airway pressure could be a major component of the repercussion of mechanical ventilation on cardiac filling. If pleural pressure variations can be considered as a fractional transmitted pressure from airway pressure, ∆P is then related to the magnitude of tidal swing of pleural pressure for given lung compliance and Vt [8, 16, 20]. This could explain why normalization of ∆PP by ∆P improved ∆PP reliability, by integrating both Vt and respiratory system compliance as opposed to normalization by tidal volume level alone (Fig. 2 of ESM).
Secondly, previous studies or comments have revealed that ∆PP can be significant without real changes in venous return but with an increase in right ventricular afterload [10, 14, 21, 22]. This second scenario occurs when patients are ventilated at relatively high lung volume above the functional residual capacity (FRC). In that case, elevated driving pressure (∆P) could reflect transpulmonary pressure variations and changes in right ventricular afterload (i.e., cyclic twist zone III to zone II West lung regions) [23–26]. Thus, positive ∆PP may not be the result of a significant change in right ventricular preload but may be due to a prevalent increase in right ventricular output impedance and afterload: Patients were thus unresponsive to fluid loading but correctly classified according to ∆PP/∆P. However, the normalization of ∆PP by ∆P could not exclude all ∆PP false positives (i.e., inspiratory elevation of pulse pressure with significant ∆up effect [20, 22] or direct transmission of pressure to the aorta [27])—but alert the clinician to be cautious if there is a significant ∆PP together with large ∆P (∆PP/∆P < 0.9).
Our results confirm previous observations on the low predictive value of ∆PP for patients ventilated with Vt < 8 ml/kg [9]. Even though a recent study found ∆PP of possible interest in ARDS patients [28], the limitations of ∆PP at low Vt in our series seem nonnegligible. Furthermore, ∆PP/∆P failed to really improve the prediction of fluid responsiveness in this population (Table 4). This unexpected result could be viewed as disappointing since the question of fluid responsiveness is of particular importance in ARDS population [29]. However, the relative high specificity compared with low sensitivity (Table 4: Spe = 88% and Sens = 43% with a 0.9 threshold value) supports the notion that, even in patients ventilated at low Vt, ∆PP/∆P could be useful to decrease the rate of false-positive ∆PPs and thus limit fluid overload. It also suggests that false-negative results observed are probably related to small respiratory variations in pleural pressure. We think that this preliminary result confirms the need to reflect on the linkage between hemodynamic and respiratory parameters in order to determine the exact performance of ∆PP/∆P, especially in patients ventilated with low Vt. As very recently outlined by Pinsky et al. [30], a universal cutoff cannot exist, and thus further physiological studies dealing with ∆PP/∆P (or other heart/lung interactions combined index [20]) are needed to better understand the respective implications of respiratory and/or circulatory processes in the genesis of stroke volume variations or its surrogate.
In any case, the present study was not designed to answer all these physiological concerns and has some limitations, with neither an echocardiographic analysis detecting LV and RV dysfunction or acute cor pulmonale, nor an assessment of pleural pressure, surrogate (i.e., oesophageal pressure) or abdominal pressure (or assessment of any increased chest wall compliance conditions).
Finally, our results show that ∆PP was not as accurate as expected to predict fluid responsiveness in comparison with previous studies [11], even in the subgroup of patients with Vt ≥ 8 ml/kg. As underscored by Perel [18], studies of fluid responsiveness are difficult to compare due to the difference in ∆PP recording methods as well as patient typology and must therefore be considered in light of several methodological specifications. For the present work we used manual recording of arterial pressure variations (see “Methods”). ∆PP was then calculated as recommended, however, at the bedside close to real-life conditions [2, 6, 7, 11]. The level of precision of this manual measurement, without increasing sampling duration [30], could explain in part the relative lack of ∆PP predictive performance, but the question of manually or automatically measured ∆PP is still a matter of debate [18]. Moreover, a case-mix phenomenon in our study population could also in part explain the modest performance of ∆PP observed, as the characteristics of medical and surgical patients and corresponding ventilatory settings differed largely from one patient to another (Table 1). Nevertheless, even if the predictive value of ∆PP in our study was quite low it performed once again better at predicting fluid responsiveness than a static marker, such as CVP, would.
In conclusion, normalizing ∆PP by using ∆PP/∆P, which is easy to calculate, was able to improve the reliability of the prediction by avoiding some of the false negatives and false positives observed by using ∆PP alone, but remained relatively inefficient at low tidal volume. This new combined index remains an interesting tool according to physiological concerns, and should encourage the clinician to analyze hemodynamic and respiratory parameters simultaneously before concluding a cardiac preload dependency, with the perspective of giving a global and comprehensive view of fluid responsiveness in the ICU.
References
Bendjelid K, Romand JA (2003) Fluid responsiveness in mechanically ventilated patients: a review of indices used in intensive care. Intensive Care Med 29:352–360
Michard F, Teboul JL (2002) Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest 121:2000–2008
Preisman S, DiSegni E, Vered Z, Perel A (2002) Left ventricular preload and function during graded haemorrhage and retranfusion in pigs: analysis of arterial pressure waveform and correlation with echocardiography. Br J Anaesth 88:716–718
Tavernier B, Makhotine O, Lebuffe G, Dupont J, Scherpereel P (1998) Systolic pressure variation as a guide to fluid therapy in patients with sepsis-induced hypotension. Anesthesiology 89:1313–1321
Berkenstadt H, Margalit N, Hadani M, Friedman Z, Segal E, Villa Y, Perel A (2001) Stroke volume variation as a predictor of fluid responsiveness in patients undergoing brain surgery. Anesth Analg 92:984–989
Michard F, Boussat S, Chemla D, Anguel N, Mercat A, Lecarpentier Y, Richard C, Pinsky MR, Teboul JL (2000) Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med 162:134–138
Michard F (2005) Changes in arterial pressure during mechanical ventilation. Anesthesiology 103:419–428 quiz 449-415
Jardin F, Genevray B, Brun-Ney D, Bourdarias JP (1985) Influence of lung and chest wall compliances on transmission of airway pressure to the pleural space in critically ill patients. Chest 88:653–658
De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent JL (2005) Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med 31:517–523
Jardin F, Delorme G, Hardy A, Auvert B, Beauchet A, Bourdarias JP (1990) Reevaluation of hemodynamic consequences of positive pressure ventilation: emphasis on cyclic right ventricular afterloading by mechanical lung inflation. Anesthesiology 72:966–970
Monnet X, Teboul JL (2007) Volume responsiveness. Curr Opin Crit Care 13:549–553
Feihl F, Broccard AF (2008) Interactions between respiration and systemic hemodynamics. Part I: basic concepts. Intensive Care Med 35:45–54
Feihl F, Broccard AF (2008) Interactions between respiration and systemic hemodynamics. Part II: practical implications in critical care. Intensive Care Med 35:198–205
Jardin F, Vieillard-Baron A (2003) Right ventricular function and positive pressure ventilation in clinical practice: from hemodynamic subsets to respirator settings. Intensive Care Med 29:1426–1434
Fessler HE (1997) Heart-lung interactions: applications in the critically ill. Eur Respir J 10:226–237
Pinsky M (2001) Hemodynamic effects of ventilation and ventilatory maneuvers. In: Scharf SM, Pinsky MR, Magder S (eds) Respiratory-circulatory interactions in health and disease. New York, Marcel Dekker Inc., pp 183–218
Pinsky MR (2004) Using ventilation-induced aortic pressure and flow variation to diagnose preload responsiveness. Intensive Care Med 30:1008–1010
Perel A (2008) Automated assessment of fluid responsiveness in mechanically ventilated patients. Anesth Analg 106:1031–1033
Romand JA, Shi W, Pinsky MR (1995) Cardiopulmonary effects of positive pressure ventilation during acute lung injury. Chest 108:1041–1048
Perel A, Minkovich L, Preisman S, Abiad M, Segal E, Coriat P (2005) Assessing fluid-responsiveness by a standardized ventilatory maneuver: the respiratory systolic variation test. Anesth Analg 100:942–945
Jardin F (2004) Cyclic changes in arterial pressure during mechanical ventilation. Intensive Care Med 30:1047–1050
Vieillard-Baron A, Chergui K, Augarde R, Prin S, Page B, Beauchet A, Jardin F (2003) Cyclic changes in arterial pulse during respiratory support revisited by Doppler echocardiography. Am J Respir Crit Care Med 168:671–676
Luecke T, Pelosi P (2005) Clinical review: positive end-expiratory pressure and cardiac output. Crit Care 9:607–621
West JB, Dollery CT, Naimark A (1964) Distribution of blood flow in isolated lung; relation to vascular and alveolar pressures. J Appl Physiol 19:713–724
Permutt S, Howell JB, Proctor DF, Riley RL (1961) Effect of lung inflation on static pressure–volume characteristics of pulmonary vessels. J Appl Physiol 16:64–70
Permutt S, Bromberger-Barnea B, Bane HN (1962) Alveolar pressure, pulmonary venous pressure, and the vascular waterfall. Medicina thoracalis 19:239–260
Denault AY, Gasior TA, Gorcsan J 3rd, Mandarino WA, Deneault LG, Pinsky MR (1999) Determinants of aortic pressure variation during positive-pressure ventilation in man. Chest 116:176–186
Huang CC, Fu JY, Hu HC, Kao KC, Chen NH, Hsieh MJ, Tsai YH (2008) Prediction of fluid responsiveness in acute respiratory distress syndrome patients ventilated with low tidal volume and high positive end-expiratory pressure. Crit Care Med 36:2810–2816
Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF, Hite RD Jr, Harabin AL (2006) Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 354:2564–2575
Kim HK, Pinsky MR (2008) Effect of tidal volume, sampling duration, and cardiac contractility on pulse pressure and stroke volume variation during positive-pressure ventilation. Crit Care Med 36:2858–2862
Acknowledgements
The authors wish to thank Joaquim Mateo M.D. for his help in preparing the manuscript and for his statistical advice, and Christophe Sugden M.Eng. for his editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is discussed in the editorial available at: doi:10.1007/s00134-009-1479-3.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vallée, F., Richard, J.C.M., Mari, A. et al. Pulse pressure variations adjusted by alveolar driving pressure to assess fluid responsiveness. Intensive Care Med 35, 1004–1010 (2009). https://doi.org/10.1007/s00134-009-1478-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-009-1478-4