Abstract
Purpose
To critically review data on the prevalence of depressive symptoms in general intensive care unit (ICU) survivors, risk factors for these symptoms, and their impact on health-related quality of life (HRQOL).
Methods
We conducted a systematic review using Medline, EMBASE, Cochrane Library, CINAHL, PsycINFO, and a hand-search of 13 journals.
Results
Fourteen studies were eligible. The median point prevalence of “clinically significant” depressive symptoms was 28% (total n = 1,213). Neither sex nor age were consistent risk factors for post-ICU depression, and severity of illness at ICU admission was consistently not a risk factor. Early post-ICU depressive symptoms were a strong risk factor for subsequent depressive symptoms. Post-ICU depressive symptoms were associated with substantially lower HRQOL.
Conclusions
Depressive symptoms are common in general ICU survivors and negatively impact HRQOL. Future studies should address how factors related to individual patients, critical illness and post-ICU recovery are associated with depression in ICU survivors.
Similar content being viewed by others
Introduction
With advances in critical care medicine, more patients are surviving intensive care unit (ICU) stays [1]. With this increase in survival, critical care research has begun focusing on long-term outcomes of ICU patients, including mental health, health-related quality of life (HRQOL), and cognitive outcomes [2–5].
Critical illness and requisite ICU therapies expose patients to enormous stressors including respiratory insufficiency, discomfort with endotracheal tube suctioning, activation of the inflammatory cascade, strain on the hypothalamic–pituitary–adrenal (HPA) axis, administration of exogenous catecholamines, and delirium with associated psychotic experiences, all within the context of a limited ability to communicate and reduced autonomy. Moreover, ICU survivors may face significant physical limitations during recovery [1]. Thus, symptoms of depression are a potential concern in ICU survivors. The recognition of depressive symptoms following critical illness is important because depression carries a risk of suicide, negatively impacts HRQOL [6], and delays return to work [7]. We previously conducted a systematic review of psychiatric morbidity in survivors of acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) and found that the point prevalence of “clinically significant” depressive symptoms ranged from 17 to 43% [8]. We are unaware of any comparable systematic review of depression in a general population of ICU survivors. In this report, we present results of a systematic review of: (1) the prevalence of depression following general ICU treatment, (2) potential risk factors for post-ICU depressive symptoms, and (3) the relationship of post-ICU depressive symptoms to HRQOL.
Methods
Search strategy
We searched Medline (1966–2007), EMBASE (1974–2007), CINAHL (1982–2007), the Cochrane Library (2007, Issue 1), and PsycInfo (1967–2007) as of 26 October 2007. Our search strategy included the following terms mapped to the appropriate MeSH/EMTREE subject headings and “exploded”: (“mental disorders” or “psychometrics”) and (“respiratory distress syndrome, adult” or “critical care” or “critical illness” or “intensive care units” or “sepsis”). The following terms were also included as text words: (“depress*” or “stress” or “anxi*”) and (“respiratory distress syndrome” or “ARDS” or “acute lung injury” or “ALI”). In addition, we manually searched the tables of contents of 13 general medicine, psychiatry, and critical care journals (see Appendix 1) from January 2000 to October 2007. The search was limited to English-language articles. Articles dealing with neonatal or pediatric intensive care were excluded.
Since we recently conducted a separate systematic review of posttraumatic stress disorder (PTSD) following ICU care [9], we did not include studies which focused exclusively on PTSD and/or non-specific anxiety outcomes. Moreover, we did not include studies focusing exclusively on ALI/ARDS survivors since psychopathology in ALI/ARDS survivors has been specifically addressed in another systematic review [8]. Our goal for this systematic review was to address areas which are not explored in these prior publications. However, related search terms from these prior systematic reviews were included to help identify several eligible, but otherwise unidentified, studies.
Study selection
Two authors (D.S.D. and S.V.D.) independently and sequentially reviewed citations, abstracts, and full-text articles to select eligible studies. All citations or abstracts selected by either author were included in the next step of the selection process. At each step, the authors calculated interobserver agreement using percent agreement and the kappa statistic. Disagreements regarding eligibility of full-text articles were resolved by consensus among all authors.
Articles were selected for review if they met the following criteria: (1) the study population was comprised of adult ICU survivors, and (2) depression assessments were conducted using validated measures at ≥1 month following ICU discharge. Abstracts, case reports, and review articles were excluded. Since we recently systematically reviewed studies focusing solely on survivors of ALI/ARDS [8], we excluded these studies from the current report. Studies focusing exclusively on survivors from specialty ICUs (i.e., coronary, trauma/surgical or neurological ICUs) were excluded in order to allow a primary focus on a more general ICU patient population and to reduce confounding from other potential risk factors for depression (e.g., myocardial infarction, head injury), recognizing that residual confounding would remain since general ICUs also care for patients with potentially confounding conditions.
Data abstraction and study quality
For each eligible study, two authors (D.S.D. and J.M.G.) independently abstracted information regarding characteristics of the study cohorts, study quality, depression measures, potential risk factors, and associations between depressive symptoms and HRQOL. The authors calculated interobserver agreement for data abstraction using percent agreement and the kappa statistic. Authors of eligible studies were contacted for additional study data (e.g., mean/median scores on depression measures), when necessary.
Study quality was assessed using five criteria adapted from the United States Preventative Task Force [10] and a previous systematic review of heterogeneous outcome data [3]: (1) enrollment of consecutive patients; (2) no loss to follow-up of >10% of study participants prior to first depressive symptoms assessment; (3) description of patients lost to follow-up; (4) at least one statistical comparison between patients lost to follow-up and those remaining in the study; and (5) adjustment for confounders by stratification, statistical adjustment, or comparison with a matched population. Quality criteria were not used in decisions regarding inclusion or exclusion of eligible studies.
Results
Search results, study characteristics and quality
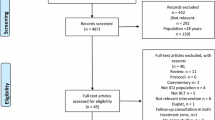
The authors reviewed 16,301 citations, 1,908 abstracts and 193 full-text articles (Fig. 1). Fourteen articles were eligible for data abstraction [11–24] (Table 1). The percent agreement (kappa statistic) for each stage of the study selection and data abstraction process were: citation review, 91% (κ = 0.54); abstract review 98% (κ = 0.84); full-text review 99% (κ = 0.89); and data abstraction, 98.7% (κ = 0.98).
Table 1 shows baseline descriptive data for the 14 studies, ordered by timing of follow-up assessments. Follow-up periods ranged from 2 weeks to approximately 14 months. The studies enrolled 1,621 unique patients. Of the 14 studies reviewed, 8 were prospective cohort studies [11–15, 18, 20, 22], one was a retrospective cohort study [21], three were cross-sectional studies [16, 19, 24], one was a case–control study [23], and one was a randomized-controlled trial [17]. Seven of the studies were conducted in the UK [13, 14, 17, 19–21, 24], five in the US [11, 16, 18, 22, 23], one in Australia [15], and one in Sweden [12].
A majority of studies enrolled patients admitted consecutively to the ICU [11, 12, 14, 15, 17, 19, 21, 22], although only six of these studies lost <10% of patients prior to the first assessment of depressive symptoms [12, 13, 16, 17, 19, 20] (Table 2). Over half of the studies that reported loss to follow-up described the patients lost to follow-up [11, 12, 14–16, 21, 24]; however, only four of these included a statistical comparison between the patients lost to follow-up and those that completed the study [11, 12, 14, 15]. Only half of the 14 studies adjusted for potential confounders in analyses [11, 13, 14, 17–19, 24].
Prior psychiatric history
Five of the studies excluded patients with a pre-ICU history of psychiatric illness [11–13, 17, 19] (Table 1). In three of these, patients were excluded only if they had a pre-existing psychotic illness or were admitted after a suicide attempt [12, 13, 17]; in the other two, patients with any prior or ongoing psychiatric illness [19] or any major psychiatric illness [11] were excluded.
Three of the studies reported information regarding patients’ psychiatric histories [13, 18, 23] (Table 3). In one of these, a recent history of depression was identified via proxy report of depressed mood most of the day for 2 weeks in the month before ICU, or by determining if the patient had been prescribed an antidepressant medication in the 6 months immediately prior to ICU admission [18]; the other two studies apparently determined prior psychiatric history through chart review alone [13, 23]. The prevalence of pre-ICU psychiatric disorders was 18% [13] and 22% [23] in two of these studies; in the third, 27% of patients had a proxy-reported recent depressed mood, 34% had been seen for psychiatric problems in their lifetime, and 33% had recently been prescribed an antidepressant [18].
Prevalence of depressive symptoms/disorder
At least one measure of depressive symptoms was completed by 1,213 patients. Ten of the studies utilized in-person assessments [11, 13, 14, 16, 17, 19–23]; two, mailed questionnaires [15, 24]; and two, a combination of in-person and telephone assessments [12, 18]. One study employed a clinician to make diagnoses using the Structured Clinical Interview for DSM-IV (SCID), a semi-structured psychiatric diagnostic interview, in addition to a questionnaire [18] (Table 3); the remaining 13 studies only used questionnaires to assess depressive symptoms. Eight of these studies utilized the Hospital Anxiety and Depression Scale (HADS) [12–14, 17, 19–21, 24]; four, the Center for Epidemiologic Studies Depression Scale (CES-D) [15, 16, 18, 22]; one, the Geriatric Depression Rating Scale-Short Form (GDS-SF) [11]; and one, the Beck Depression Inventory-II (BDI-II) [23].
In determining the median point prevalence of questionnaire-ascertained “clinically significant” depressive symptoms post-ICU across studies, there were two important challenges. First, some studies collected depressive symptom data at more than one time point; in those instances, we used the median value from each study. Second, some studies had treatment or case groups versus control groups; in those instances, we used the prevalence for the entire sample, reasoning that the control groups in these studies are not necessarily more representative than the treatment or case groups. Notably, of the eight studies employing the HADS, two employed a more stringent threshold to define “clinically significant” depressive symptoms (i.e., a score >11, rather than the more common threshold of >8). The median point prevalence of “clinically significant” depressive symptoms post-ICU was 28% (range 8–57%) (14 studies, n = 1,213). The point prevalence of any clinician-diagnosed depressive disorder 2 months post-ICU was 33% (1 study, n = 134) [18]. In the five studies that explicitly tested for the effect of time on symptoms of depression [12, 14, 15, 18, 20], three found significant decreases in mean/median depression scores during the first 2–12 months post-ICU [12, 14, 15] (Table 3).
Potential risk factors for depressive symptoms/disorder
Patient-specific factors
Only one [14] of three studies [14, 18, 21] (n = 80–153) found a significant association between female sex and post-ICU depressive symptoms (measured at hospital discharge, Table 4). None of three studies found an association between age and depression [14, 18, 20] (n = 51–153).
Only one study examined premorbid psychopathology or physical functioning as a risk factor for post-ICU depressive symptoms [18]. Proxy-reported depressed mood in the month before ICU predicted depressive symptoms at 2 and 6 months post-ICU, though antidepressant prescription in the 6 months before ICU did not predict post-ICU depressive symptoms. Poor pre-ICU physical functioning also predicted post-ICU depressive symptoms [18].
ICU illness and treatment factors
Only one study examined ICU admission diagnosis as a risk factor for post-ICU depression; no association was evident [13]. ICU length of stay was not a significant predictor in any of the three studies [13, 14, 20]. Severity of illness at ICU admission, as measured by the Acute Physiology and Chronic Health Evaluation II (APACHE II) score, was not a significant predictor in any of the three studies [13, 14, 20]. The in-ICU duration of sedation [13] and interruption of sedation [23] were not associated with post-ICU depressive symptoms.
Early post-ICU memories of in-ICU experiences
Three studies examined early post-ICU memories of in-ICU experiences as predictors of depressive symptoms. Poor recall of the ICU at hospital discharge, but not memories of frightening experiences, predicted depressive symptoms 6 months post-ICU in one study [14]. Memories of “extremely stressful” in-ICU nightmares or fearfulness 5 days post-ICU predicted depressive symptoms 2 months post-ICU in another study [12]. Memories of in-ICU psychotic/nightmare experiences were cross-sectionally associated with more depressive symptoms 14 days after ICU discharge in one study [13].
Post-ICU psychiatric symptoms
Psychiatric symptoms at hospital discharge and beyond were prospective predictors or cross-sectional correlates of post-ICU depressive symptoms in five of five studies [11, 12, 14, 18, 20]. Depressive symptoms at hospital discharge were a strong predictor of depressive symptoms at both 6 and 12 months post-ICU in one study [14], and depressive symptoms at 2 months post-ICU were a strong predictor of depressive symptoms at 6 months post-ICU in another study [18]. Post-ICU PTSD symptoms were strongly correlated cross-sectionally with post-ICU depressive symptoms in two studies [12, 20]; post-ICU non-specific anxiety symptoms were also strongly correlated cross-sectionally with post-ICU depressive symptoms in the one study that examined this issue [20]. Finally, cognitive impairment at 6 months was associated with more depressive symptoms in one study that examined this association [11].
Post-ICU physical functioning
Post-ICU physical functioning was examined as a correlate of post-ICU depressive symptoms in two studies [18, 20]. Although change in physical functioning from hospital discharge to 2 months post-ICU was not a significant predictor of post-ICU depressive symptoms, change in physical functioning from 2 to 6 months post-ICU was a significant predictor of depressive symptoms at 6 months in one study [18]. In the other study, increased physical symptoms, as measured by a non-validated symptom scale, were significant predictors of depressive symptoms at 3 and 9 months post-ICU [20].
Association of depressive symptoms/disorder and health-related quality of life
While eight studies evaluated HRQOL [11, 15, 16, 18–20, 22, 23], only two studies examined the relationships between depressive symptoms and HRQOL [18, 20] (Table 5). Symptoms of depression were associated with lower HRQOL, as measured with the Medical Outcomes Study Short Form-36 (SF-36) [25], in both studies. In one study [18], diagnoses of SCID incident major depressive episode or depressive disorder not otherwise specified were associated with a lower SF-36 physical functioning domain score. In the other study [20], symptoms of depression were more strongly associated with mental health HRQOL domains measured via the mental component summary score than with physical health HRQOL domains measured via the physical component summary score, although associations with the latter were also substantial.
Discussion
This systematic review of depressive symptoms in general ICU survivors highlights three important issues. First, the prevalence of substantial depressive symptoms is quite high in the year after ICU care. Across studies, the median point prevalence of questionnaire-ascertained “clinically significant” depressive symptoms was 28% (14 studies, n = 1,213), and the point prevalence of clinician-diagnosed depressive disorder was 33%. These prevalences are substantially higher than the 7% 1-year prevalence of major depressive disorder in a recent study of US adults that utilized non-clinicians administering a structured interview [26], and are also considerably greater than the 8 and 4% prevalences of “clinically significant” depressive symptoms ascertained in non-clinical samples using two of the same questionnaires described in this study, the HADS and the GDS-SF, utilizing similar cutoffs for case definition [27, 28]. In addition, these figures are higher than the median point prevalence of substantial depressive symptoms in survivors of acute myocardial infarction (14% in hospital, lower thereafter) [29] in studies using the HADS with the lower threshold (score > 8) for case definition, and are also higher than the point prevalence of these symptoms in a recent systematic review of burn injury survivors (4–13% using the HADS with variable thresholds for case definition) [30]. The median point prevalence of “clinically significant” depressive symptoms reported here is similar to that in survivors of ALI/ARDS [8]. Notably, our median point prevalence estimate is likely an underestimate for the population of all general ICU survivors since several of the studies excluded patients with pre-ICU psychopathology.
Second, although ten studies examined potential risk factors for post-ICU depressive symptoms, many of the specific risk factors examined were unique to particular studies. Nevertheless, in cases where similar risk factors were considered across multiple studies, a few conclusions can be drawn: (1) neither sex nor age were consistent risk factors; (2) severity of illness at ICU admission was consistently not a risk factor; and (3) early post-ICU depressive symptoms were a strong risk factor for subsequent post-ICU depressive symptoms. Interestingly, although ICU treatment factors such as duration of sedation and ICU length of stay may be associated with increased depressive symptoms in ALI survivors [8], similar associations were not evident in the three studies that examined this issue in our review [13, 14, 20].
Third, post-ICU depressive symptoms may have substantial associations with both physical and mental health aspects of quality of life on HRQOL. Though only two studies addressed this issue, the results are consistent with findings in ALI/ARDS survivors [8], and with the general literature on the effect of depression on HRQOL [6, 31]. More detailed discussions of HRQOL after critical illness and measurement instruments can be found in prior publications [3, 4]; additional research is needed to understand the nature of these associations, as well as to understand other associations between depressive symptoms post-ICU and function. For instance, there is extensive research demonstrating that untreated depression is associated with worse outcomes for patients with chronic medical illnesses and with lower adherence to treatment regimens for these diseases [32]; similar research in patients surviving critical illnesses would be valuable.
The existing literature has several important limitations. First, only a modest percentage of patients eligible to participate in these studies did so, introducing the potential for selection bias. Also, 13 of the 14 studies relied exclusively on questionnaires (i.e., screening instruments) to estimate the burden of depressive symptoms in-ICU survivors. To our knowledge, only the CES-D has been validated against clinician diagnoses in the post-ICU setting [18]. Future studies of depressive symptoms in ICU survivors should utilize expert clinicians to conduct diagnostic interviews in larger cohorts, to more definitively estimate prevalence and incidence, and to evaluate the psychometric properties of other existing questionnaires (e.g., the HADS, which has been used most commonly) in this setting. In addition, the mental health domains of the SF-36 quality of life instrument may measure some of the same symptoms as the depression measures [33]. Hence, it may be difficult to know if an association between depressive symptoms and mental health aspects of quality of life reflect overlapping constructs; nevertheless, there are also associations between depressive symptoms and physical health aspects of quality of life, though the directionality of any cause–effect relationship is unclear.
Additional research is necessary to understand pre-ICU risk factors for post-ICU depression. Since prior anxiety and depressive disorders are potent risk factors for depression [34], it is striking that only one study examined survivors’ pre-ICU psychopathology (specifically, depression shortly before ICU admission) as a risk factor for post-ICU depression [18]. Remarkably, no study evaluated personality traits as a predictor of post-ICU depressive symptoms. High neuroticism (a general tendency to experience negative emotions) is a strong predictor of depression in the setting of stress [35], and future studies should explicitly assess this variable as a potential risk factor.
Potential ICU-related risk factors for post-ICU depression also require further research. Weinert [36] hypothesized that post-ICU depression is due to neuronal changes in the context of critical illness. However, potential etiologic factors such as hypoxia, inflammatory cytokines, or stress on the hypothalamic–pituitary–adrenal axis have not been examined in this context. The finding that critical illness-induced cognitive impairment is associated with depressive symptoms [11] may support the hypothesis of direct neuronal involvement in post-ICU depression. Also consistent with this hypothesis, our group recently found that in-ICU hypoglycemia was a predictor of depressive symptoms at 3 months after ALI/ARDS [37]. In addition, delirium is very common in the ICU [38], and patients may be at greater risk for depression following episodes of delirium [39]. Factors related to physical health and function, such as ICU-acquired weakness, may also play a role in mental health outcomes. Further research is essential in order to clarify the role that these and other ICU-related factors may play.
In addition, post-ICU factors should be considered in any comprehensive model of post-ICU depression. Based on the limited available research, post-ICU depression may be associated with post-ICU physical functioning [18, 20]. Also, PTSD, which is common following ICU stays [9], may affect the development of depressive symptoms; depression and PTSD can frequently co-occur following a traumatic event [40], and major depressive disorder is the most frequent concomitant psychiatric condition in patients with PTSD [41]. Other factors, such as chronic medical illnesses, family/financial strain, as well as feelings of loss due to post-ICU cognitive and physical deficits, could also add risk for post-ICU depression; such factors should be examined in future studies.
The number of studies examining psychiatric morbidity following ICU stays has steadily increased over the last decade. Given this trend and the findings in this systematic review, we make several recommendations for future studies of psychopathology after critical illness. First, where possible, studies assessing the prevalence of psychiatric disorders post-ICU should utilize expert clinicians administering semi-structured diagnostic interviews, and such studies should also attempt to validate existing questionnaires against interview results. Second, assessments should be carried out at more than one time point to evaluate the natural history of post-ICU psychopathology. Third, future studies should assess pre-ICU psychiatric illness and personality traits as risk factors for post-ICU psychopathology and incorporate these factors into a comprehensive model, including in-ICU factors (e.g., sedatives) and post-ICU factors (e.g., physical and cognitive deficits).
Several potential limitations of this systematic review are noteworthy. First, confidence regarding the precision and validity of our findings should be tempered given the methodological limitations of the original studies, as described above. Second, although we summarized evidence that depressive symptoms may decrease over time after ICU discharge [12, 14, 15], we did not feel we could draw strong conclusions regarding the effect of time on prevalence of substantial depressive symptoms given the small number and heterogeneity of the existing studies. Third, although we excluded studies that focused exclusively on survivors from trauma, neurological, coronary, or surgical specialty ICUs, some patients in the reviewed studies did have trauma, neurologic, cardiac and surgical problems which may be important independent risk factors for depression; thus, we cannot conclude that the reported high prevalences of depressive symptoms are specific to critical illness and ICU treatment alone. Fourth, many of the specific risk factors reviewed were unique to particular studies, without external replication, limiting their generalizability. Future research in this field will benefit from new, more comprehensive reviews and studies of risk factors for depression. Moreover, it may be advantageous to specifically review studies of survivors from specialty ICUs to obtain a more complete understanding of risk factors and to evaluate if risk factors vary substantially by type of critical illness. Finally, despite a comprehensive search of 16,301 citations, potentially eligible studies may have been inadvertently omitted due to inconsistent indexing of eligible studies in electronic databases.
In conclusion, depressive symptoms are common in general ICU survivors and may negatively impact HRQOL. Although further research is necessary to more fully define risk factors for post-ICU depression, we can preliminarily conclude that sex, age and severity of illness at ICU admission are not risk factors, and early post-ICU depressive symptoms are a strong risk factor for subsequent depressive symptoms. Clinicians should recognize that symptoms of depression are common following intensive care, requiring collaboration between intensivists, primary care physicians, and psychiatrists to ensure prompt, comprehensive evaluation and treatment in order to reduce post-ICU morbidity and improve ICU survivors’ quality of life.
Abbreviations
- ICU:
-
Intensive care unit
References
Angus DC, Carlet J (2003) Surviving intensive care: a report from the 2002 Brussels Roundtable. Intensive Care Med 29:368–377
Broomhead LR, Brett SJ (2002) Clinical review: intensive care follow-up—what has it told us? Crit Care 6:411–417
Dowdy DW, Eid MP, Sedrakyan A, Mendez-Tellez PA, Pronovost PJ, Herridge MS, Needham DM (2005) Quality of life in adult survivors of critical illness: a systematic review of the literature. Intensive Care Med 31:611–620
Dowdy DW, Eid MP, Dennison CR, Mendez-Tellez PA, Herridge MS, Guallar E, Pronovost PJ, Needham DM (2006) Quality of life after acute respiratory distress syndrome: a meta-analysis. Intensive Care Med 32:1115–1124
Hopkins RO, Jackson JC (2006) Long-term neurocognitive function after critical illness. Chest 130:869–878
Wells KB, Stewart A, Hays RD, Burnam MA, Rogers W, Daniels M, Berry S, Greenfield S, Ware J (1989) The functioning and well-being of depressed patients: results from the Medical Outcomes Study. JAMA 262:914–919
Zatzick DF, Jurkovich GJ, Rivara FP, Wang J, Fan MY, Joesch J, MacKenzie E (2008) A national study of posttraumatic stress disorder, depression, and work and functional outcomes after hospitalization for traumatic injury. Ann Surg 248:429–437
Davydow DS, Desai SV, Needham DM, Bienvenu OJ (2008) Psychiatric morbidity in survivors of the acute respiratory distress syndrome: a systematic review. Psychosom Med 70:512–519
Davydow DS, Gifford JM, Desai SV, Needham DM, Bienvenu OJ (2008) Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen Hosp Psychiatry 30:421–434
Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM, Atkins D (2001) Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med 20:21–35
Jackson JC, Hart RP, Gordon SM, Shintani A, Truman B, May L, Ely EW (2003) Six-month neuropsychological outcome of medical intensive care unit patients. Crit Care Med 31:1226–1234
Samuelson KAM, Lundberg D, Fridlund B (2007) Stressful memories and psychological distress in adult mechanically ventilated intensive care unit patients—a 2-month follow-up study. Acta Anaesthesiol Scand 51:671–678
Jones C, Griffiths RD, Humphris G, Skirrow PM (2001) Memory, delusions, and the development of acute posttraumatic stress disorder-related symptoms after intensive care. Crit Care Med 29:573–580
Rattray JE, Johnston M, Wildsmith JA (2005) Predictors of emotional outcomes of intensive care. Anaesthesia 60:1085–1092
Boyle M, Murgo M, Adamson H, Gill J, Elliott D, Crawford M (2004) The effect of chronic pain on health related quality of life amongst intensive care unit survivors. Aust Crit Care 17:104–113
Guentner K, Hoffman LA, Happ MB, Kim Y, Devito Dabbs A, Mendelsohn AB, Chelluri L (2006) Preferences for mechanical ventilation among survivors of prolonged mechanical ventilation and tracheostomy. Am J Crit Care 15:65–77
Jones C, Skirrow P, Griffiths RD, Humphris GH, Ingleby S, Eddleston J, Waldmann C, Gager M (2003) Rehabilitation after critical illness: a randomized, controlled trial. Crit Care Med 31:2456–2461
Weinert C, Meller W (2006) Epidemiology of depression and antidepressant therapy after acute respiratory failure. Psychosomatics 47:399–407
Young E, Eddleston J, Ingleby S, Streets J, McJanet L, Wang M, Glover L (2005) Returning home after intensive care: a comparison of symptoms of anxiety and depression in ICU and elective cardiac surgery patients and their relatives. Intensive Care Med 31:86–91
Sukantarat K, Greer S, Brett S, Williamson R (2007) Physical and psychological sequelae of critical illness. Br J Health Psychol 12:65–74
Eddleston JM, White P, Guthrie E (2000) Survival, morbidity, and quality of life after discharge from intensive care. Crit Care Med 28:2293–2299
Chelluri L, Kyung AI, Belle SH, Schulz R, Rotondi AJ, Donahoe MP, Sirio CA, Mendelsohn AB, Pinsky MR (2004) Long-term mortality and quality of life after prolonged mechanical ventilation. Crit Care Med 32:61–69
Kress JP, Gehlbach B, Lacy M, Pliskin N, Pohlman AS, Hall JB (2003) The long-term psychological effects of daily sedative interruption on critically ill patients. Am J Resp Crit Care Med 168:1457–1461
Scragg P, Jones A, Fauvel N (2001) Psychological problems following ICU treatment. Anaesthesia 56:9–14
Ware JE Jr, Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30:473–483
Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE (2005) Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:617–627
Crawford JR, Henry JD, Crombie C, Taylor EP (2001) Normative data for the HADS from a large non-clinical sample. Br J Clin Psychol 40:429–434
Ferraro FR, Chelminski I (1996) Preliminary normative data on the Geriatric Depression Scale-Short Form (GDS-SF) in a young adult sample. J Clin Psychol 52:443–447
Thombs BD, Bass EB, Ford DE, Stewart KJ, Tsilidis KK, Patel U, Fauerbach JA, Bush DE, Ziegelstein RC (2005) Prevalence of depression in survivors of acute myocardial infarction. J Gen Intern Med 21:30–38
Thombs BD, Bresnick MG, Magyar-Russell G (2006) Depression in survivors of burn injury: a systematic review. Gen Hosp Psychiatry 28:494–502
Coryell W, Scheftner W, Keller M, Endicott J, Maser J, Klerman GL (1993) The enduring psychosocial consequences of mania and depression. Am J Psychiatry 150:720–727
Cassano P, Fava M (2002) Depression and public health: an overview. J Psychosom Res 53:849–857
Katschnig H (1997) How useful is the concept of quality of life in psychiatry? Curr Opin Psychiatry 10:337–345
Kendler KS, Gardner CO, Prescott CA (2006) Toward a comprehensive developmental model for major depression in men. Am J Psychiatry 163:115–124
Kendler KS, Kuhn J, Prescott CA (2004) The interrelationship of neuroticism, sex, and stressful life events in the prediction of episodes of major depression. Am J Psychiatry 161:631–636
Weinert C (2005) Epidemiology and treatment of psychiatric conditions that develop after critical illness. Curr Opin Crit Care 11:376–380
Dowdy DW, Dinglas V, Mendez-Tellez PA, Bienvenu OJ, Sevransky J, Dennison CR, Shanholtz C, Needham DM (2008) Intensive care unit hypoglycemia predicts depression during early recovery from acute lung injury. Crit Care Med 36:2726–2733
Pisani MA, McNichol L, Inouye SK (2003) Cognitive impairment in the intensive care unit. Clin Chest Med 24:727–737
Davydow DS (2009) Symptoms of depression and anxiety following delirium: a review. Psychosomatics
Nixon RDV, Resick PA, Nishith P (2004) An exploration of comorbid depression among female victims of intimate partner violence with posttraumatic stress disorder. J Affect Disord 82:315–320
Foa EB, Stein DJ, MacFarlane AC (2006) Symptomatology and psychopathology of mental health problems after disaster. J Clin Psychiatry 67(Suppl 2):15–25
Acknowledgments
The authors thank R.N. Martin Boyle and R.N. Christina Jones, Ph.D., for providing additional data from their studies included in this review. Dr. Needham is supported by a Clinician-Scientist Award from the Canadian Institutes of Health Research and by the National Institutes of Health (Acute Lung Injury SCCOR Grant # P050 HL73994). Dr. Bienvenu is supported by a Career Development Award from the National Institutes of Health (K23 MH64543). The funding bodies had no role in the study design, manuscript writing or decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Appendix 1: Manually searched journals from January 2000 to October 2007
Appendix 1: Manually searched journals from January 2000 to October 2007
-
American Journal of Critical Care
-
American Journal of Psychiatry
-
American Journal of Respiratory and Critical Care Medicine
-
Archives of General Psychiatry
-
Biological Psychiatry
-
Critical Care
-
Critical Care Medicine
-
General Hospital Psychiatry
-
Intensive Care Medicine
-
JAMA
-
New England Journal of Medicine
-
Psychosomatic Medicine
-
Psychosomatics
Rights and permissions
About this article
Cite this article
Davydow, D.S., Gifford, J.M., Desai, S.V. et al. Depression in general intensive care unit survivors: a systematic review. Intensive Care Med 35, 796–809 (2009). https://doi.org/10.1007/s00134-009-1396-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-009-1396-5