Abstract
Aims/hypothesis
IL-1β and TNF-α contribute to pancreatic beta cell death in type 1 diabetes. Both cytokines activate the transcription factor nuclear factor-κB (NF-κB), but recent observations suggest that NF-κB blockade prevents IL-1β + IFN-γ- but not TNF-α + IFN-γ-induced beta cell apoptosis. The aim of the present study was to compare the effects of IL-1β and TNF-α on cell death and the pattern of NF-κB activation and global gene expression in beta cells.
Methods
Cell viability was measured after exposure to IL-1β or to TNF-α alone or in combination with IFN-γ, and blockade of NF-κB activation or protein synthesis. INS-1E cells exposed to IL-1β or TNF-α in time course experiments were used for IκB kinase (IKK) activation assay, detection of p65 NF-κB by immunocytochemistry, real-time RT-PCR and microarray analysis.
Results
Blocking NF-κB activation protected beta cells against IL-1β + IFNγ- or TNFα + IFNγ-induced apoptosis. Blocking de novo protein synthesis did not increase TNF-α- or IL-1β-induced beta cell death, in line with the observations that cytokines induced the expression of the anti-apoptotic genes A20, Iap-2 and Xiap to a similar extent. Microarray analysis of INS-1E cells treated with IL-1β or TNF-α showed similar patterns of gene expression. IL-1β, however, induced a higher rate of expression of NF-κB target genes putatively involved in beta cell dysfunction and death and a stronger activation of the IKK complex, leading to an earlier translocation of NF-κB to the nucleus.
Conclusions/interpretation
NF-κB activation in beta cells has a pro-apoptotic role following exposure not only to IL-1β but also to TNF-α. The more marked beta cell death induced by IL-1β is explained at least in part by higher intensity NF-κB activation, leading to increased transcription of key target genes.
Similar content being viewed by others
Introduction
Proinflammatory cytokines such as IL-1β, TNF-α and IFN-γ are secreted by activated macrophages and T cells in the vicinity of beta cells during insulitis, contributing to beta cell dysfunction and death [1–3]. Beta cell exposure to IL-1β in vitro induces functional impairment and, in combination with IFN-γ and/or TNF-α, causes cell death, mostly by apoptosis [2, 3]. Apoptosis is regulated by extracellular signals, intracellular ATP levels, phosphorylation cascades and expression of diverse pro- and anti-apoptotic gene networks. Although both cytokines activate the same key transcription factor, namely, nuclear factor-κB (NF-κB), IL-1β has a more pronounced pro-apoptotic effect than TNF-α in beta cells [4]. NF-κB activation plays an important role in cytokine-induced beta cell death, since NF-κB inhibition in vitro protects against IL-1β + IFN-γ [5–7], while conditional and beta cell-specific NF-κB blockade in vivo prevents the development of diabetes in response to multiple low doses of streptozotocin [7]. In beta cells, NF-κB controls diverse gene networks that modulate its phenotype, attract and activate immune cells, and contribute to apoptosis by ER stress [8–11] and other, as yet unknown, mechanisms [3]. Of note, NF-κB has mostly anti-apoptotic effects in other cell types [4, 12, 13], and recent data suggest that TNF-α-induced activation of NF-κB in beta cells is protective [14].
Each NF-κB complex is composed of one or two members of a family of five proteins that form homo- or heterodimers in a cell type- and stimulus-specific manner [15–18]. These dimers are usually kept in the cytoplasm bound to a group of inhibitor κB proteins (IκBs) [17, 18]. Stimulation by proinflammatory cytokines results in IκB phosphorylation mediated by the IκB kinase (IKK) complex, leading to IκB degradation and translocation of NF-κB to the nucleus [17, 18]. The frequency and duration of activation of specific NF-κB dimers, together with their transactivation capacity, determine the regulation of specific sets of genes and particular cellular responses [4, 15, 16]. The effect of NF-κB on transcription is also modulated by post-translational mechanisms, such as phosphorylation of the p65 subunit by mitogen-activated protein kinases, IKK, Akt and NF-κB-activating kinase [17–19]. In beta cells, extracellular signal-regulated kinase (ERK), which is activated by IL-1β but not TNF-α [4, 20], increases the transactivation capacity of NF-κB via phosphorylation of the p65 subunit [21].
Since TNF-α and IL-1β activate NF-κB and, in combination with IFN-γ, induce beta cell death [2], they are believed to trigger similar signal transduction pathways and target genes. These cytokines, however, act via different receptors, recruiting distinct adaptor molecules and kinase cascade pathways [17]. The TNF-α receptor can also induce apoptosis via the caspase pathway, but this is usually prevented by the expression of NF-κB-dependent anti-apoptotic genes [22–24].
In the present study we used array analysis and biological studies of key pathways to determine the pattern of NF-κB activation and gene expression induced by IL-1β and TNF-α in beta cells, to clarify the molecular mechanisms underlying the different effects produced by these cytokines.
Methods
Cell culture
Pancreatic islets were isolated from adult Wistar rats (Charles River Laboratories, Brussels, Belgium) and used in accordance with the Belgian Regulations for Animal Care and the Ethics Committee for Animal Experiments of the ULB. Beta cells were purified by FACS (FACStar; Becton-Dickinson, Sunnyvale, CA, USA) [25, 26]. After purification, preparations contained 93 ± 3% beta cells (n = 6). Experiments requiring large number of cells were performed with the well-differentiated INS-1E cells [27], kindly provided by C. Wolheim (Center Medical Universitaire, Geneva, Switzerland). Cytokines induce a similar pattern of gene expression in INS-1E cells as in primary beta cells, but INS-1E cells are more sensitive to cytokine-induced cell death [28, 29]. INS-1E (passage 57-73) and rat fibroblast 208F cells (European Collection Cell Cultures [ECACC], Salisbury, UK) were cultured as previously described [4].
Cell treatment
The following cytokine concentrations were used, based on previous dose–response experiments [2, 4, 28]: recombinant human IL-1β (a kind gift from C. W. Reinolds, National Cancer Institute, Bethesda, MD, USA) at 50 U/ml (rat primary beta cells), 10 or 100 U/ml (INS-1E and 208F cells); recombinant murine TNF-α (Innogenetics, Gent, Belgium) at 1,000 U/ml, and recombinant rat IFN-γ (R&D systems, Abingdon, UK) at 0.036 μg/ml. In some experiments cells were preincubated with cycloheximide (1 μg/ml) for 2 h, which inhibits 90% of protein synthesis [30]. Cell medium was collected for nitrite determination [28, 31]. The inducible nitric oxide synthase (iNOS) blocker N G-methyl-l-arginine (LMA; Sigma, Steinheim, Germany) was used at a concentration of 1 mmol/l [28].
Infection with recombinant adenoviruses
Cells were left uninfected (control) or infected either with AdLUC (luciferase-expressing virus) or AdIκB(SA)2 (a virus expressing an NF-κB super repressor protein) [6, 8, 32]. Cells were infected for 2 h at 37°C with a multiplicity of infection (MOI) of ten for purified rat beta cells and 6.5 for INS-1E cells. The MOIs were selected based on lowest toxicity by viral infection combined with highest blockade of NF-κB activation. After infection (24–48 h), cells were treated with cytokines. We have previously shown that infection of beta cells with AdIκB(SA)2 at the MOIs used in the present study does not change its function [6].
Assessment of cell viability and caspase-3 activity
The percentages of viable, necrotic and apoptotic cells were determined by staining with Hoechst 33342/propidium iodide [4, 28, 33], and the apoptotic index calculated [4]. NunView 488 Caspase-3 Kit for live cells (Biotium, Hayward, CA, USA) was used for determination of caspase-3 activation. Cellular DNA was stained with Hoechst 342 (20 μg/ml) [33] and then cells were incubated with caspase-3 substrate for at least 15 min. Caspase-3 substrate cleavage stains the nucleus of positive cells green (ultraviolet excitation at 450–490 nm). Caspase-3-positive cells were calculated as a percentage of total number of Hoechst-stained cells (ultraviolet excitation at 365–380 nm).
Determination of NF-κB activation by immunofluorescence
Cells were fixed with 4% (vol./vol.) paraformaldehyde, permeabilised with 70% acetone/30% methanol (vol./vol.), and incubated for 1 h with anti-p65 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or the negative control anti-haemagglutinin (Roche Diagnostics, Mannheim, Germany) at 1:500 dilution. Secondary antibody FITC-conjugated anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA, USA; diluted 1/200) was used for visualisation by inverted fluorescence microscopy (Axiovert 200, Zeiss, Oberkochen, Germany). NF-κB activation was evaluated by the presence of p65 in the nucleus; the percentage of positive cells was determined in at least 500 cells for each experimental condition.
Microarray analysis
Total RNA was isolated from INS-1E and 208F cells using the RNeasy Mini Kit (Qiagen, Venlo, the Netherlands). The aminoallyl antisense-cRNA was obtained from total RNA, then coupled to one of two fluorophores—indocarbocyanine (Cy3) or indodicarbocyanine (Cy5) (Amersham, Diegem, Belgium)—and prepared for hybridisation [34]. Hybridisation using the APOCHIP array [34] was performed in duplicate, with dye swapping, as previously described [35]. Microarray scanning and quantification using the GenePix Pro 5.0 image analysis software (Axon Instruments, Union City, CA, USA), gene expression Log2 ratios calculated from the spot intensities, and normalisation of the data were performed as previously described [35]. Means for the three independent experiments were used for a one sample Student’s t test. Gene expression was considered as changed by cytokines when p ≤ 0.01 and fold change (up- or downregulated) ≥1.2.
Real-time quantitative RT-PCR analysis
Cells were harvested and the reverse transcriptase reaction was performed using poly(A) RNA [9]. Expression of the NF-κB target genes Mcp-1, Iκbα, Cd40, A20, Xiap (also known as Ccl2, Nfkbia, Tnfrsf5, Tnfaip3, Birc4, respectively) and Iap-2 was determined by real-time RT-PCR [9]. Expression values are corrected for Gapdh, a housekeeping gene, and values are shown as fold induction relative to control (no cytokine) [4]. Cytokines do not modify Gapdh expression in insulin-producing cells [9, 28, 29]. Primer sequences and their respective PCR fragment lengths are provided in Table 1 of the Electronic supplementary material (ESM).
IKK immunoprecipitation and kinase assay
Cells (106) were lysed in buffer containing 25 mmol/l HEPES pH 7.9; 150 mmol/l NaCl; 0.5% Triton X-100; 10% glycerol; 1 mmol/l dithiothreitol (DTT); 1 mmol/l Na3VO4; 25 mmol/l β-glycerophosphate; 1 mmol/l NaF and protease inhibitor cocktail (Roche Diagnostics). Total protein (500–700 μg) was rotated for 2 h at 4°C in the presence of 1.2 μg of either anti-IKKγ (Santa Cruz Biotechnology) or anti-haemagglutinin (Roche Diagnostics) antibodies, followed by a 2 h incubation with protein A-sepharose CL-4B beads (GE Healthcare, Uppsala, Sweden). Beads were washed twice with lysis buffer, once with kinase buffer (25 mmol/l HEPES, pH 7.9, 10 mmol/l MgCl2, 25 mmol/l β-glycerophosphate, 1 mmol/l DTT, 1 mmol/l Na3VO4), and then incubated at 30°C in kinase buffer containing 1 μg of glutathione-S-transferase (GST)-IκBα recombinant protein and 5 mmol/l ATP. After 30 min, the reaction was stopped by heat denaturation in the presence of 1% (wt/vol.) SDS. Proteins were subjected to SDS-PAGE through a 10% (wt/vol.) gel and transferred onto a nitrocellulose membrane. Immunoblot analysis was performed with anti-phospho (P)-IκBα and anti-IKKα (Santa Cruz Biotechnology). Primary antibodies were detected with anti-rabbit horseradish peroxidase-labelled anti-IgG (Pierce Protein Research Products; Thermo Scientific, Rockford, IL, USA).
Promoter reporter assays
Cells were co-transfected using lipofectamine 2000 (Invitrogen, Baesley, UK) with the internal control pRL-CMV encoding Renilla luciferase (Promega, Madison, WI, USA) and with either the pNF-κB-Luciferase (BD Biosciences, Palo Alto, CA, USA), or the control vector pTAL [4]. Luciferase activities and values correction performed as described previously [10]. After transfection (16 h), cells were exposed to cytokines for 4 h.
Statistical analysis
Data are shown as means ± SEM, and comparisons between groups were carried out either by paired Student’s t test (two-tailed) or by ANOVA followed by paired Student’s t test with the Bonferroni correction, as indicated. A p value of ≤0.05 was considered statistically significant.
Results
IL-1β- and TNF-α-induced NF-κB activation is pro-apoptotic in beta cells
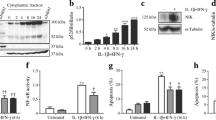
Blocking NF-κB activation by infection with AdIκBα(SA)2 protected purified rat beta cells and INS-1E cells against both IL-1β + IFN-γ- and TNF-α + IFNγ-induced cell death (Fig. 1a,b), but protection was more marked in primary beta cells than in INS-1E cells. Consistent with this, infection with AdIκBα(SA)2 provided more effective prevention of NF-κB nuclear localisation in primary beta cells than in INS-1E cells (Fig. 1c,d). In beta cells, cytokine-induced expression of the gene encoding iNOS is regulated by NF-κB [36], and in line with the findings for viability and NF-κB nuclear localisation, inhibition of nitric oxide (NO) production in response to AdIκBα(SA)2 infection was more pronounced in primary beta cells than in INS-1E cells (Fig. 1e,f). Cytokine-induced beta cell death (Fig. 1a,b), NF-κB nuclear localisation (Fig. 1c,d) and NO formation (Fig. 1e,f) were not prevented in the control cells infected with AdLUC, confirming that the findings in the AdIκBα(SA)2-infected cells were due to blockade of NF-κB activation.
NF-κB inhibition prevents cytokine-induced beta cell apoptosis. FACS-purified rat beta cells (a, c, e) or insulin-producing INS-1E cells (b, d, f) were left uninfected (control, white bars), or infected with recombinant adenoviruses expressing either luciferase (AdLUC, grey bars) or the NF-κB non-degradable inhibitor IκB(SA)2 [AdIkB(SA)2, black bars]. a, b Viability assay: cells were left untreated or exposed to cytokines for 4 days (purified rat beta cells, a) or 48 h (INS-1E cells, b) and viability was measured as described in Methods. Results are expressed as apoptotic index [4] vs respective control (not cytokine-treated). The percentages of apoptotic cells in controls (not cytokine-treated) were 13 ± 1.3%, 14 ± 1% and 17 ± 2% for beta cells, and 5.5 ± 0.5%, 7 ± 1% and 9 ± 1% for INS-1E cells for uninfected, AdLUC-infected and AdIκBα(SA)2-infected cells, respectively. c, d Immunofluorescence assay for the NF-κB p65 subunit after 1 h of exposure to IL-1β or TNF-α; the figures represent the percentage of nuclear NF-κB-positive cells. e, f Nitrite production measured in the medium collected from cells used in the viability assay shown in a and b. Results are the mean ± SEM of four to nine experiments. *p < 0.05 vs uninfected cells (paired Student’s t test)
IL-1β + IFN-γ and TNF-α + IFN-γ induced INS-1E cell death mostly via apoptosis, with a minor necrotic component. Prevention of NO production by LMA decreased the apoptosis induced by both treatments (ESM Fig. 1a), although this did not return to control levels, in spite of near-complete inhibition of nitrite production (ESM Fig. 1b). Prevention of cell death by LMA was more pronounced for the cells treated with IL-1β + IFN-γ (ESM Fig. 1a), in agreement with the higher NO production induced by this treatment (ESM Fig. 1b).
Role of protein synthesis in cytokine-induced beta cell death and caspase-3 activation
Blocking protein synthesis induced primary beta cell and INS-1E cell apoptosis, but this effect was not, or only marginally, increased by IL-1β or TNF-α (Fig. 2a,b). Similar findings were observed when the effect of the two cytokines on caspase-3 activation was measured (Fig. 2c,d). When these experiments were repeated in 208F cells, in which NF-κB is anti-apoptotic [4], TNF-α + cycloheximide treatment induced a significant increase in cell death (19.5% ± 0.8; n = 4, p ≤ 0.001 vs TNF-α alone) compared with TNF-α (2.9% ± 0.5, n = 4) or cycloheximide alone (5.4% ± 1.1, n = 4).
Apoptosis induction in beta cells exposed to IL-1β (IL) or TNF-α (TNF), alone (white bars) or in combination with cycloheximide (CH, grey bars). Cytokines were added for 24 h to INS-1E cells (b, d) or for 48 h to purified rat beta cells (a, c) after pre-treatment with CH for 2 h (CH remained in the medium). a, b Percentage of apoptotic cells, as measured by Hoechst 33342/propidium iodide. c, d Percentage of cells with caspase-3 activation. Results are the mean ± SEM of three to eight independent experiments. *p ≤ 0.05 vs control without CH, **p ≤ 0.01 treated cells vs respective control (+ or − CH), by ANOVA
Identification of genes modulated by IL-1β and TNF-α by microarray analysis
Three independent time course experiments using INS-1E or 208F cells treated with IL-1β or TNF-α were used for array analysis. In total, the analysis detected 490 (INS-1E) and 479 genes (208F cells), represented by 875 spots (each spot containing a different probe) and flagged as ‘good’ for the GenePix Pro 5.0 software. These genes were clustered in 14 groups [29] according to their putative biological function (ESM Tables 2 and 3). Cytokines modified the expression of 39 genes in INS-1E cells (Table 1). Most of these genes are related to cytokine processing and signal transduction or to transcription factors. Comparison of these data with the available information on IL-1β-induced gene expression in purified rat beta cells ([29] and Beta Cell Gene Expression Bank at http://t1dbase.org/page/BCGB_Enter/display/, accessed 18 March 2008), showed 78% similarity. The gene expression patterns induced by IL-1β and TNF-α were similar (Table 1), but the expression of the majority of upregulated genes, particularly the putative NF-κB target genes, was higher following IL-1β treatment (Table 1, Fig. 3a–d). Interestingly, the converse was seen in 208F cells, i.e. induction of these genes was greater in response to TNF-α than to IL-1β (ESM Table 3, Fig. 3e–h). Real-time RT-PCR was performed on independent time course experiments in INS-1E cells to confirm the APOCHIP results for some of the cytokine-modulated genes (Fig. 4). The qualitative changes in gene expression observed by real-time RT-PCR were in good agreement with the observed results for the APOCHIP. Thus, the cytokines increased Iκbα and Mcp-1 expression, IL-1β to a greater extent than TNF-α (Figs 3 c,d and 4 a,b), and no significant induction of Xiap or Iap-2 (ESM Table 2, Fig. 4c,d). The quantitative changes, however, were lower in the APOCHIP than those observed by real-time RT-PCR (Figs 3 and 4). These differences were reported in previous array analyses [35, 37], and may be an innate limitation of the array technology. The expression of additional NF-κB target genes that were not present (A20) or detected (Cd40) in the APOCHIP was also analysed. Induction of these genes was again greater in INS-1E cells following IL-1β treatment compared with TNF-α treatment (Fig. 4e,f). Of note, A20, Xiap and Iap-2 are anti-apoptotic genes regulated by NF-κB [24].
Microarray results of cytokine-induced expression of the NF-κB target genes Mob1 (a, e), Bax (b, f), Mcp-1 (c, g) and Iκbα (d, h) in INS-1E (a–d) and 208F (e–h) cells. Cells were exposed to IL-1β (100 U/ml, diamonds) or TNF-α (1,000 U/ml, squares). Results are the mean ± SEM of fold variations vs controls for three independent microarray experiments
Real-time RT-PCR analysis of the NF-κB target genes Mcp-1 (a), Iκbα (b), Xiap (c), Iap-2 (d), A20 (e) and Cd40 (f) in INS-1E cells exposed to cytokines. The mRNA was extracted from cells treated with 100 U/ml IL-1β (diamonds) or 1,000 U/ml TNF-α (squares). Results were corrected for Gapdh expression and are shown as fold induction compared with control values. Results are the mean ± SEM of three to four independent experiments. *p ≤ 0.05 IL-1β vs TNF-α, by paired Student’s t test
IL-1β induces an earlier and more pronounced NF-κB translocation to INS-1E cell nucleus than TNF-α
Exposure of INS-1E cells to IL-1β or TNF-α induced p65 (a NF-κB member present in the cytokine-activated complex in INS-1E cells [4]) translocation to the nucleus (Fig. 5); this was more marked for IL-1β at the earlier time points (between 10 and 30 min). Between 45 min and 2 h, NF-κB nuclear localisation tended to be similar between the two treatments, but at later time points, i.e. between 4 and 48 h, IL-1β again induced greater NF-κB nuclear localisation.
Temporal analysis of NF-κB nuclear localisation induced by IL-1β (100 U/ml) or TNF-α (1,000 U/ml) in INS-1E cells. Cells were exposed to IL-1β (white bars) or TNF-α (black bars) at the indicated time points. NF-κB activation was measured by the presence of p65 (NF-κB subunit) in the cell nucleus by immunocytochemistry assay. Results are the mean ± SEM of six independent experiments. *p < 0.05 IL-1β vs TNF-α, paired Student’s t test
Differential IKK activation induced by cytokines in INS-1E cells
In INS-1E cells, IKK activation by IL-1β was stronger and longer than that by TNF-α (Fig. 6a), as measured by the capacity of the immunoprecipitated IKK complex to phosphorylate the GST-IκBα substrate. On the other hand, and in line with the array data (Fig. 3), IKK activation by TNF-α was more marked in 208F cells (Fig. 6b). The absence of phosphorylation when lysates were immunoprecipitated by anti-haemagglutinin antibody, used as a negative control, confirmed that the GST-IκBα substrate is specifically phosphorylated by the IKK complex (Fig. 6a,b). Immunoprecipitation of the IKK complex was confirmed by western blotting using anti-IKKα antibody, indicating a similar amount of IKKα protein (a key member of the IKK complex) as immunoprecipitated by anti-IKKγ (Fig. 6a,b). In agreement with these findings, IL-1β induced a stronger NF-κB activation, as evaluated by a NF-κB reporter assay (fold increase: 32.6 ± 5.7, p ≤ 0.05 vs control, paired Student’s t test, n = 4) compared with TNF-α (fold increase: 12.2 ± 1.8, p ≤ 0.05 vs control, paired Student’s t test, n = 3). In contrast, TNF-α and IL-1β induced similar NF-κB activation in 208F cells, with a tendency for a higher degree of activation with TNF-α (fold increase: 2.4 ± 0.5, p ≤ 0.05 vs control, paired Student’s t test, n = 9) compared with IL-1β (fold increase: 1.9 ± 0.3, p ≤ 0.05 vs control, paired Student’s t test, n = 9).
Differential activation of the IKK complex by IL-1β (100 U/ml) or TNF-α (1,000 U/ml). After a time course treatment with the cytokines, INS-1E (a) and 208F (b) cells were lysed and the IKK complex was immunoprecipitated (IP) with anti-IKKγ antibody. IKK activation was measured by its capacity to phosphorylate the substrate glutathione-S-transferase (GST)-IκBα. Western blot for IKKα showed similar IP of the IKK complex. Anti-haemagglutinin (HA) antibody was used as a negative control for IP. The figure is representative of three to four similar experiments
Discussion
Understanding the mechanisms by which IL-1β and TNF-α trigger beta cell death is important for the development of novel strategies to protect these cells in type 1 diabetes. In pancreatic beta cells, IL-1β has a more pronounced pro-apoptotic effect than TNF-α, but both cytokines activate the transcription factor NF-κB [4]. Recent observations suggest that NF-κB activation by IL-1β or TNF-α may lead to different beta cell outcomes, with a pro-apoptotic role for IL-1β [5–7] and an apparent anti-apoptotic role, perhaps mediated by de novo synthesis of A20 [38], for TNF-α [14]. In the present study we evaluated the mechanisms regulating IL-1β- and TNF-α-induced beta cell apoptosis, focusing on NF-κB. Neither IL-1β nor TNF-α induces primary beta cell death on its own, and these cytokines usually act in synergy with IFN-γ during insulitis [1, 2]. Thus, most experiments aiming to determine beta cell death were performed in the presence of IFN-γ. Blockade of NF-κB activation by AdIκBα(SA)2 protected beta cells from both IL-1β + IFN-γ- and TNF-α + IFN-γ-induced apoptosis. Protection was, however, partial, indicating the presence of additional non-NF-κB-mediated mechanisms of cell death. The increase in apoptosis stimulated by blockade of protein synthesis was not further increased in the presence of IL-1β or TNF-α in beta cells, but was markedly increased by TNF-α in fibroblasts. In line with these findings, the two cytokines failed to induce expression of the anti-apoptotic NF-κB target genes Iap-2 and Xiap, and induced only a transient expression of A20 in INS-1E cells (Fig. 4).
In other cell types, TNF-α induces an anti-apoptotic response that is dependent on NF-κB activation and de novo gene expression/protein production [22–24]. This explains why inhibition of protein synthesis sensitises fibroblasts to TNF-α-induced cell death ([22–24], present data). In beta cells, however, this anti-apoptotic response is either of minor magnitude or is overshadowed by the pro-apoptotic signals. Thus, there was no increase in cell death when beta cells were exposed to TNF-α or IL-1β in the presence of cycloheximide. The discrepancy between the present observations and previous data, showing increased cell death in the insulinoma cell line β-TC3 [38], may be due to the use of tumoral cells in these previous experiments. Indeed blocking of protein synthesis in combination with TNF-α in INS-1E cells (an insulinoma cell line) induced a minor increase in cell death as compared to cycloheximide alone, while no such effect was observed in primary beta cells (Fig. 2a).
The present findings are in agreement with previous studies underlying the deleterious effects of TNF-α in beta cells [2, 39] but do not support the suggestion that TNF-α-induced NF-κB activation has a protective role against beta cell death [14]. It is conceivable that the discrepant results between this and previous studies may be, at least in part, related to the use of different experimental models. Prevention of NF-κB activation induces a clearer protection against cytokine-induced cell death in primary beta cells than in INS-1E cells (present data). INS-1E cells have a doubling time of 66–72 h, while purified primary beta cells have very limited proliferative capacity in vitro. INS-1E cells were exposed to cytokines 48 h after viral infection and viability was measured after an additional 48 h in culture; this timing is sufficient for 1.4 cycles of cell division, which might have “diluted” the viral vector. In line with this possibility we observed a higher inhibition of NF-κB nuclear localisation in INS-1E cells analysed 24 h after infection (82%) compared with 48 h (57%) (data not shown).
Activation of NF-κB in beta cells in vivo may also have a pro- or anti-apoptotic function depending on the animal model of diabetes and time window utilised. Thus, there is a pro-apoptotic effect of NF-κB in the case of multiple low doses of streptozotocin [7] while NF-κB has an apparent anti-apoptotic effect in the case of NOD mice [14]. Duration of NF-κB inhibition may also be relevant, since protection against diabetes was reported with temporary and conditional inactivation [7] while prolonged and constitutive inhibition aggravated the disease [14]. Since there are different mediators of beta cell death in vivo [2], it is difficult to define from these experiments which pathway(s) (e.g. IL-1β, TNF-α, Fas, iNOS) is being modulated by NF-κB.
IL-1β + TNFα + IFNγ induces NF-κB activation in whole human islets [40], and it will be of interest to study this in individual humans beta cells. Moreover, and taking into account the growing interest in beta cell neogenesis, it will be of relevance to evaluate whether NF-κB has a similar pro-apoptotic role in proliferating/differentiating beta cells.
We next explored the reason(s) for the more intense pro-apoptotic effect of IL-1β compared with TNF-α, even though it was used at a tenfold lower concentration [4]. We first analysed the role of NO and then examined the global pattern of gene expression induced by TNF-α and IL-1β. The enzyme responsible for NO formation, iNOS, is induced by proinflammatory cytokines in beta cells and contributes to beta cell death in rodents [2, 3]. IL-1β + IFN-γ induces higher NO production than TNF-α + IFN-γ ([2], present data). Blocking iNOS activity with LMA only partially prevented the cell death induced by both treatments, which was more marked for IL-1β + IFN-γ (40%) than for TNF-α + IFN-γ (15%). This suggests that both IL-1β + IFN-γ- [28, 41] and TNF-α + IFN-γ-induced INS-1E cell death have NO-dependent and independent components. The NO-independent component is apparently more relevant for TNF-α + IFN-γ-induced cell death.
The global pattern of gene expression induced by TNF-α and IL-1β was compared in INS-1E cells, using our ‘home-made’ APOCHIP array [34]. IL-1β and TNF-α modified the expression of similar sets of genes, and the majority of the modified genes were either related to cytokine expression, processing and signal transduction (30%) or were transcription factors (18%). IL-1β, however, induced a higher expression of genes related to beta cell dysfunction and death, and most of these genes have been previously described as NF-κB targets [8, 42]. The opposite was observed in fibroblast 208F cells, where gene expression was induced to a greater extent by TNF-α than IL-1β. The APOCHIP is a focused array, designed to include apoptosis-related and/or cytokine-regulated genes [34]. Thus, we cannot exclude the possibility that relevant genes that were not included in the APOCHIP are differentially regulated by IL-1β and TNF-α.
Previous experiments have shown that IL-1β-induced NF-κB activation in INS-1E cells is more rapid, intense and sustained than in fibroblasts, leading to a more pronounced activation of key downstream genes such as iNOS and Fas [4]. This indicates that the intensity of NF-κB activation and the cell type involved are important for the pro- or anti-apoptotic outcome. This is supported by the observation that transient and limited activation of NF-κB induced by extracellular matrix favours preservation of rat beta cell function and survival [43]. In the present experiments, the higher expression of IL-1β- compared with TNF-α-induced genes was paralleled by an earlier and more marked nuclear localisation of NF-κB. This is probably secondary to the stronger IKK activation induced by IL-1β. Both IL-1β and TNF-α induce NF-κB activation through the IKK-IκB signalling pathway [17, 18]. They act, however, via different receptors and recruit different protein adaptors [17]. The IKK complexes are heterogeneous [44], and the different responses of INS-1E cells to IL-1β and TNF-α may be related to kinetically distinct pathways of IKK activation and IκBα degradation, as shown in monocytes [45]. TNF-α signalling via TNF receptor 1 in other cells results in the rapid activation of IKK and nearly complete degradation of IκBα within 10 min [17]. In 208F cells (present data), IKK activation was increased to a greater extent by TNF-α than IL-1β, demonstrating that INS-1E cells possess a specific pattern of cytokine-induced IKK activation. IKK augments the transcriptional activity of NF-κB via phosphorylation of the p65 subunit in some cell types [19]. Post-translational modifications modulate both the strength and duration of the NF-κB response [46], and in INS-1E cells transcriptional activity is higher following exposure to IL-1β than TNF-α, as observed in reporter gene assays ([4], present data). IL-1β treatment also increases the activation of ERK1/2 [4], which regulates the trans-activating capacity of NF-κB in insulin-producing cells [21] and contributes to cytokine-induced beta cell death [47].
Figure 7 provides an overview of the main findings of this study. Some of the differences between the deleterious effects of IL-1β and TNF-α in beta cells might be explained by the higher intensity of IKK activation and consequent NF-κB nuclear migration induced by IL-1β. This leads to increased NO production, in parallel with the augmented expression of other genes, such as Fas and Mcp-1, which will contribute to both increased apoptosis and attraction of immune-competent cells [2]. Beta cell sensitivity to cytokines is aggravated by its absent/feeble and/or transitory induction of protective genes such as Xiap [48], Bcl-6 [49] and A20 [38] (present observations). Overall, our findings allow the following conclusions: (1) IL-1β and TNF-α share a main common pathway of signal transduction in beta cells, namely, NF-κB activation; (2) NF-κB activation is mostly pro-apoptotic in beta cells exposed to either IL-1β or TNF-α; (3) the effects of IL-1β on NF-κB are more intense than those of TNF-α, which may explain why IL-1β induces more apoptosis. However, we cannot exclude the possibility that other, yet to be discovered, pathways also contribute to the effects of these cytokines.
Abbreviations
- ERK:
-
extracellular signal-regulated kinase
- GST:
-
glutathione S-transferase
- IκB:
-
inhibitor κB
- IKK:
-
IκB kinase
- iNOS:
-
inducible nitric oxide synthase
- LMA:
-
N G-methyl-l-arginine
- NF-κB:
-
nuclear factor κB
- NO:
-
nitric oxide
References
Thomas HE, Kay TW (2000) Beta cell destruction in the development of autoimmune diabetes in the non-obese diabetic (NOD) mouse. Diabetes Metab Res Rev 16:251–261
Eizirik DL, Mandrup-Poulsen T (2001) A choice of death—the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia 44:2115–2133
Cnop M, Welsh N, Jonas JC et al (2005) Mechanisms of pancreatic beta cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 54(Suppl 2):S97–S107
Ortis F, Cardozo AK, Crispim D, Storling J, Mandrup-Poulsen T, Eizirik DL (2006) Cytokine-induced pro-apoptotic gene expression in insulin-producing cells is related to rapid, sustained and non-oscillatory NF-κB activation. Mol Endocrinol 20:1867–1879
Giannoukakis N, Rudert WA, Trucco M, Robbins PD (2000) Protection of human islets from the effects of interleukin-1β by adenoviral gene transfer of an IκB repressor. J Biol Chem 275:36509–36513
Heimberg H, Heremans Y, Jobin C et al (2001) Inhibition of cytokine-induced NF-κB activation by adenovirus-mediated expression of a NF-κB super-repressor prevents beta cell apoptosis. Diabetes 50:2219–2224
Eldor R, Yeffet A, Baum K et al (2006) Conditional and specific NF-κB blockade protects pancreatic beta cells from diabetogenic agents. Proc Natl Acad Sci U S A 103:5072–5077
Cardozo AK, Heimberg H, Heremans Y et al (2001) A comprehensive analysis of cytokine-induced and nuclear factor-κB-dependent genes in primary rat pancreatic beta-cells. J Biol Chem 276:48879–48886
Cardozo AK, Ortis F, Storling J et al (2005) Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic beta cells. Diabetes 54:452–461
Pirot P, Ortis F, Cnop M et al (2007) Transcriptional regulation of the endoplasmic reticulum stress gene Chop in pancreatic insulin-producing cells. Diabetes 56:1069–1077
Eizirik DL, Cardozo AK, Cnop M (2008) The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev 29:42–61
Karin M, Lin A (2002) NF-κB at the crossroads of life and death. Nat Immunol 3:221–227
Dutta J, Fan Y, Gupta N, Fan G, Gelinas C (2006) Current insights into the regulation of programmed cell death by NF-κB. Oncogene 25:6800–6816
Kim S, Millet I, Kim HS et al (2007) NF-κB prevents beta cell death and autoimmune diabetes in NOD mice. Proc Natl Acad Sci U S A 104:1913–1918
Hoffmann A, Leung TH, Baltimore D (2003) Genetic analysis of NF-κB/Rel transcription factors defines functional specificities. EMBO J 22:5530–5539
Saccani S, Pantano S, Natoli G (2003) Modulation of NF-κB activity by exchange of dimers. Mol Cell 11:1563–1574
Hayden MS, Ghosh S (2004) Signaling to NF-κB. Genes Dev 18:2195–2224
Hayden MS, Ghosh S (2008) Shared principles in NF-κB signaling. Cell 132:344–362
Vermeulen L, De Wilde G, Notebaert S, Van den Berghe W, Haegeman G (2002) Regulation of the transcriptional activity of the nuclear factor-κB p65 subunit. Biochem Pharmacol 64:963–970
Andersen NA, Larsen CM, Mandrup-Poulsen T (2000) TNFα and IFNγ potentiate IL-1β induced mitogen activated protein kinase activity in rat pancreatic islets of Langerhans. Diabetologia 43:1389–1396
Larsen L, Storling J, Darville M et al (2005) Extracellular signal-regulated kinase is essential for interleukin-1-induced and nuclear factor κB-mediated gene expression in insulin-producing INS-1E cells. Diabetologia 48:2582–2590
Beg AA, Baltimore D (1996) An essential role for NF-κB in preventing TNF-α-induced cell death. Science 274:782–784
Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM (1996) Suppression of TNF-α-induced apoptosis by NF-κB. Science 274:787–789
Muppidi JR, Tschopp J, Siegel RM (2004) Life and death decisions: secondary complexes and lipid rafts in TNF receptor family signal transduction. Immunity 21:461–465
Pipeleers DG, in’t Veld PA, Van de Winkel M, Maes E, Schuit FC, Gepts W (1985) A new in vitro model for the study of pancreatic A and B cells. Endocrinology 117:806–816
Rasschaert J, Ladriere L, Urbain M et al (2005) Toll-like receptor 3 and STAT-1 contribute to double-stranded RNA + interferon-γ-induced apoptosis in primary pancreatic beta-cells. J Biol Chem 280:33984–33991
Janjic D, Maechler P, Sekine N, Bartley C, Annen AS, Wolheim CB (1999) Free radical modulation of insulin release in INS-1 cells exposed to alloxan. Biochem Pharmacol 57:639–648
Kutlu B, Cardozo AK, Darville MI et al (2003) Discovery of gene networks regulating cytokine-induced dysfunction and apoptosis in insulin-producing INS-1 cells. Diabetes 52:2701–2719
Cardozo AK, Kruhoffer M, Leeman R, Orntoft T, Eizirik DL (2001) Identification of novel cytokine-induced genes in pancreatic beta-cells by high-density oligonucleotide arrays. Diabetes 50:909–920
Cnop M, Ladriere L, Hekerman P et al (2007) Selective inhibition of eukaryotic translation initiation factor 2 alpha dephosphorylation potentiates fatty acid-induced endoplasmic reticulum stress and causes pancreatic beta-cell dysfunction and apoptosis. J Biol Chem 282:3989–3997
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and nitrate in biological fluids. Anal Biochem 126:131–138
Jobin C, Panja A, Hellerbrand C et al (1998) Inhibition of proinflammatory molecule production by adenovirus-mediated expression of a nuclear factor κB super-repressor in human intestinal epithelial cells. J Immunol 160:410–418
Hoorens A, Stange G, Pavlovic D, Pipeleers D (2001) Distinction between interleukin-1-induced necrosis and apoptosis of islet cells. Diabetes 50:551–557
Magnusson NE, Cardozo AK, Kruhoffer M, Eizirik DL, Orntoft TF, Jensen JL (2005) Construction and validation of the APOCHIP, a spotted oligo-microarray for the study of beta-cell apoptosis. BMC Bioinformatics 6:311
Pirot P, Naamane N, Libert F et al (2007) Global profiling of genes modified by endoplasmic reticulum stress in pancreatic beta cells reveals the early degradation of insulin mRNAs. Diabetologia 50:1006–1014
Darville MI, Eizirik DL (1998) Regulation by cytokines of the inducible nitric oxide synthase promoter in insulin-producing cells. Diabetologia 41:1101–1108
Dooley TP, Curto EV, Davis RL, Grammatico P, Robinson ES, Wilborn TW (2003) DNA microarrays and likelihood ratio bioinformatic methods: discovery of human melanocyte biomarkers. Pigment Cell Res 16:245–253
Liuwantara D, Elliot M, Smith MW et al (2006) Nuclear factor-κB regulates beta-cell death: a critical role for A20 in beta-cell protection. Diabetes 55:2491–2501
Mandrup-Poulsen T, Bendtzen K, Dinarello CA, Nerup J (1987) Human tumor necrosis factor potentiates human interleukin 1-mediated rat pancreatic beta-cell cytotoxicity. J Immunol 139:4077–4082
Flodstrom M, Welsh N, Eizirik DL (1996) Cytokines activate the nuclear factor kappa B (NF-kappa B) and induce nitric oxide production in human pancreatic islets. FEBS Lett 385:4–6
Liu D, Pavlovic D, Chen MC, Flodstrom M, Sandler S, Eizirik DL (2000) Cytokines induce apoptosis in beta-cells isolated from mice lacking the inducible isoform of nitric oxide synthase (iNOS−/−). Diabetes 49:1116–1122
Naamane N, van Helden J, Eizirik DL (2007) In silico identification of NF-κB-regulated genes in pancreatic beta-cells. BMC Bioinformatics 8:55
Hammar EB, Irminger JC, Rickenbach K et al (2005) Activation of NF-κB by extracellular matrix is involved in spreading and glucose-stimulated insulin secretion of pancreatic beta cells. J Biol Chem 280:30630–30637
Connelly L, Palacios-Callender M, Ameixa C, Moncada S, Hobbs AJ (2001) Biphasic regulation of NF-κB activity underlies the pro- and anti-inflammatory actions of nitric oxide. J Immunol 166:3873–3881
Nasuhara Y, Adcock IM, Catley M, Barnes PJ, Newton R (1999) Differential IκB kinase activation and IκBa degradation by interleukin-1β and tumor necrosis factor-α in human U937 monocytic cells. Evidence for additional regulatory steps in κB-dependent transcription. J Biol Chem 274:19965–19972
Chen LF, Greene WC (2004) Shaping the nuclear action of NF-κB. Nat Rev Mol Cell Biol 5:392–401
Pavlovic D, Andersen NA, Mandrup-Poulsen T, Eizirik DL (2000) Activation of extracellular signal-regulated kinase (ERK)1/2 contributes to cytokine-induced apoptosis in purified rat pancreatic beta-cells. Eur Cytokine Netw 11:267–274
Kim HS, Kim S, Lee MS (2005) IFN-γ sensitizes MIN6N8 insulinoma cells to TNF-α-induced apoptosis by inhibiting NF-κB-mediated XIAP upregulation. Biochem Biophys Res Commun 336:847–853
Kharroubi I, Lee CH, Hekerman P, Darville MI, Evans RM, Eizirik DL et al (2006) BCL-6: a possible missing link for anti-inflammatory PPAR-δ signalling in pancreatic beta cells. Diabetologia 49:2350–2358
Acknowledgements
This work has been supported by grants from the Communauté Française de Belgique—Actions de Recherche Concertées (ARC; the French Community of Belgium—Concerted Research Actions), Fonds National de la Recherche Scientifique (FNRS; National Fund for Scientific Research) Belgium, the Belgium Program on Interuniversity Poles of Attraction initiated by the Belgium State (IUAP P6/40) and European Union (STREP Savebeta, contract no. 036903 in the Framework Programme 6 of the European Community). F. Moore is the recipient of a post-doctoral fellowship from FNRS, Belgium. E. Théâtre is a research fellow at the FNRS, Belgium. A. Chariot is supported by grants from the FNRS, TELEVIE, the Belgian Association Against Cancer, the Concerted Research Action Program (04/09-323, University of Liege), the Inter-University Attraction Pole 5/12 (Federal Ministry of Science), the ‘Centre Anti-Cancéreux’ and the ‘Leon Fredericq’ Foundation (University of Liege). We thank the personnel from the Laboratory of Experimental Medicine, ULB, for excellent technical support: M. A. Neef, G. Vandenbroeck, M. Urbain, J. Schoonheydt, R. Leeman, and S. Mertens.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Supplementary Material Available Statement
The supplementary materials contain the time evolution charts of RMSD, R g, percentage of saved native contacts and α-helical content for all studied fragments.
ESM Fig. 1
Role of NO production in cytokine-induced cell death. INS-1E cells were exposed to IL-1β + IFN-γ or to TNF-α + IFN-γ in the presence (black bars) or absence (white bars) of the iNOS blocker NG-methyl-l-arginine (LMA; Sigma, Steinheim, Germany; used at a concentration of 1 mmol/l [28]) for 48 h. a Apoptosis expressed as a percentage of the total number of cells counted. b Nitrite production [28, 31]. Results are the mean ± SEM of four to six experiments. *p ≤ 0.05 vs cytokine, paired Student’s t test. (PDF 73.7 KB)
ESM Table 1
Primer sequences and their respective PCR fragment lengths (PDF 57 KB)
ESM Table 2
Complete list of genes scored as present in INS-1E cells(XLS 792 KB)
ESM Table 3
Complete list of genes scored as present in 208F cells(XLS 826 KB)
Rights and permissions
About this article
Cite this article
Ortis, F., Pirot, P., Naamane, N. et al. Induction of nuclear factor-κB and its downstream genes by TNF-α and IL-1β has a pro-apoptotic role in pancreatic beta cells. Diabetologia 51, 1213–1225 (2008). https://doi.org/10.1007/s00125-008-0999-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-008-0999-7