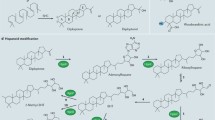
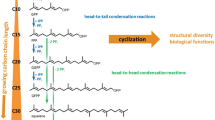
Hopanoids are pentacyclic triterpenoid lipids occurring in bacteria. They are synthesized from isopentenyl units which are formed in a new biosynthetic route leading to isopentenyl diphosphate. Six C5 units are joined to form squalene, the immediate precursor in hopanoid synthesis. In a highly complex cyclization reaction that shares considerable similarities with that of oxidosqualene to sterols, the hopane skeleton is formed from squalene by the squalene-hopene cyclase. Recent elucidation of the X-ray structure of this membrane-bound cyclase has shed some light on the properties of this unusual enzyme. The active site is located in a cavity within the enzyme. The squalene substrate diffuses through a channel structure from the membrane into this cavity and is there transformed into hopene. Polar side chains are attached to hopene resulting in the amphiphilic molecular structure of many hopanoids. These hopanoids are membrane components involved in regulating membrane fluidity and stability. However, the many structural variants of hopanoids indicate that they may have other interesting but as yet unknown functions.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kannenberg, E., Poralla, K. Hopanoid Biosynthesis and Function in Bacteria. Naturwissenschaften 86, 168–176 (1999). https://doi.org/10.1007/s001140050592
Issue Date:
DOI: https://doi.org/10.1007/s001140050592