Abstract
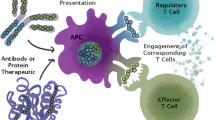
Nanotechnology offers enormous potential in drug delivery and in vivo imaging. Nanoparticles (NPs), for example, are being extensively tested as scaffolds to deliver anti-cancer therapeutics or imaging tags. Our recent work, discussed herein, indicates that an opportunity exists to use NPs to deliver ligands for, and trigger, cognate receptors on T lymphocytes as a way to induce therapeutic immune responses in vivo. Specifically, systemic delivery of NPs coated with Type 1 diabetes (T1D)-relevant peptide-major histocompatibility complex molecules triggered the expansion of cognate memory autoregulatory (disease-suppressing) T cells, suppressed the progression of autoimmune attack against insulin-producing beta cells, and restored glucose homeostasis. This therapeutic avenue exploits a new paradigm in the progression of chronic autoimmune responses that enables the rational design of disease-specific “nanovaccines” capable of blunting autoimmunity without impairing systemic immunity, a long sought-after goal in the therapy of these disorders. Here, we discuss the research paths that led to the discovery of this therapeutic avenue and highlight the features that make it an attractive approach for the treatment, in an antigen-specific manner, of a whole host of autoimmune diseases.





Similar content being viewed by others
References
Jenkins MK, Pardoll DM, Mizuguchi J, Quill H, Schwartz RH (1987) T-cell unresponsiveness in vivo and in vitro: fine specificity of induction and molecular characterization of the unresponsive state. Immunol Rev 95:113–135
Sharma SD, Nag B, Su XM, Green D, Spack E, Clark BR, Sriram S (1991) Antigen-specific therapy of experimental allergic encephalomyelitis by soluble class II major histocompatibility complex-peptide complexes. Proc Natl Acad Sci USA 88:11465–11469
Nag B, Kendrick T, Arimilli S, Yu SC, Sriram S (1996) Soluble MHC II-peptide complexes induce antigen-specific apoptosis in T cells. Cell Immunol 170:25–33. doi:10.1006/cimm.1996.0130
Nag B, Arimilli S, Mukku PV, Astafieva I (1996) Functionally active recombinant alpha and beta chain-peptide complexes of human major histocompatibility class II molecules. J Biol Chem 271:10413–10418
Arimilli S, Mumm JB, Nag B (1996) Antigen-specific apoptosis in immortalized T cells by soluble MHC class II-peptide complexes. Immunol Cell Biol 74:96–104. doi:10.1038/icb.1996.13
Goodkin DE, Shulman M, Winkelhake J, Waubant E, Andersson P, Stewart T, Nelson S, Fischbein N, Coyle PK, Frohman E, Jacobs L, Holcenberg J, Lee M, Mocci S (2000) A phase I trial of solubilized DR2:MBP84-102 (AG284) in multiple sclerosis. Neurology 54:1414–1420
Nicolle MW, Vincent A, Sharma S, Nag B, Willcox N, Newsom-Davis J (1993) An in vitro model for disease-specific immunotherapy in myasthenia gravis using soluble MHC class II bound to AChR-derived peptide. Ann NY Acad Sci 681:577–580
Nicolle MW, Nag B, Sharma SD, Willcox N, Vincent A, Ferguson DJ, Newsom-Davis J (1994) Specific tolerance to an acetylcholine receptor epitope induced in vitro in myasthenia gravis CD4+ lymphocytes by soluble major histocompatibility complex class II-peptide complexes. J Clin Invest 93:1361–1369. doi:10.1172/JCI117112
Bond AP, Corlett L, Curnow SJ, Spack E, Willcox N, Newsom-Davis J (1998) Diverse patterns of unresponsiveness in an acetylcholine receptor-specific T-cell clone from a myasthenia gravis patient after engaging the T-cell receptor with three different ligands. J Neuroimmunol 82:182–190
Spack EG, McCutcheon M, Corbelletta N, Nag B, Passmore D, Sharma SD (1995) Induction of tolerance in experimental autoimmune myasthenia gravis with solubilized MHC class II:acetylcholine receptor peptide complexes. J Autoimmun 8:787–807
Kavanaugh A, Genovese M, Baughman J, Kivitz A, Bulpitt K, Olsen N, Weisman M, Matteson E, Furst D, van Vollenhoven R, Anderson J, Cohen S, Wei N, Meijerink J, Jacobs C, Mocci S (2003) Allele and antigen-specific treatment of rheumatoid arthritis: a double blind, placebo controlled phase 1 trial. J Rheumatol 30:449–454
Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG (1997) A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 389:737–742. doi:10.1038/39614
Thornton AM, Shevach EM (1998) CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med 188:287–296
Burrows GG, Bebo BF Jr, Adlard KL, Vandenbark AA, Offner H (1998) Two-domain MHC class II molecules form stable complexes with myelin basic protein 69–89 peptide that detect and inhibit rat encephalitogenic T cells and treat experimental autoimmune encephalomyelitis. J Immunol 161:5987–5996
Burrows GG, Chang JW, Bachinger HP, Bourdette DN, Offner H, Vandenbark AA (1999) Design, engineering and production of functional single-chain T cell receptor ligands. Protein Eng 12:771–778
Burrows GG, Adlard KL, Bebo BF Jr, Chang JW, Tenditnyy K, Vandenbark AA, Offner H (2000) Regulation of encephalitogenic T cells with recombinant TCR ligands. J Immunol 164:6366–6371
Sinha S, Subramanian S, Miller L, Proctor TM, Roberts C, Burrows GG, Vandenbark AA, Offner H (2009) Cytokine switch and bystander suppression of autoimmune responses to multiple antigens in experimental autoimmune encephalomyelitis by a single recombinant T-cell receptor ligand. J Neurosci 29:3816–3823. doi:10.1523/JNEUROSCI.5812-08.2009
Wang C, Gold BG, Kaler LJ, Yu X, Afentoulis ME, Burrows GG, Vandenbark AA, Bourdette DN, Offner H (2006) Antigen-specific therapy promotes repair of myelin and axonal damage in established EAE. J Neurochem 98:1817–1827. doi:10.1111/j.1471-4159.2006.04081.x
Offner H, Subramanian S, Wang C, Afentoulis M, Vandenbark AA, Huan J, Burrows GG (2005) Treatment of passive experimental autoimmune encephalomyelitis in SJL mice with a recombinant TCR ligand induces IL-13 and prevents axonal injury. J Immunol 175:4103–4111
Huan J, Subramanian S, Jones R, Rich C, Link J, Mooney J, Bourdette DN, Vandenbark AA, Burrows GG, Offner H (2004) Monomeric recombinant TCR ligand reduces relapse rate and severity of experimental autoimmune encephalomyelitis in SJL/J mice through cytokine switch. J Immunol 172:4556–4566
Link JM, Rich CM, Korat M, Burrows GG, Offner H, Vandenbark AA (2007) Monomeric DR2/MOG-35-55 recombinant TCR ligand treats relapses of experimental encephalomyelitis in DR2 transgenic mice. Clin Immunol 123:95–104. doi:10.1016/j.clim.2006.12.002
Vandenbark AA, Rich C, Mooney J, Zamora A, Wang C, Huan J, Fugger L, Offner H, Jones R, Burrows GG (2003) Recombinant TCR ligand induces tolerance to myelin oligodendrocyte glycoprotein 35–55 peptide and reverses clinical and histological signs of chronic experimental autoimmune encephalomyelitis in HLA-DR2 transgenic mice. J Immunol 171:127–133
Sinha S, Subramanian S, Proctor TM, Kaler LJ, Grafe M, Dahan R, Huan J, Vandenbark AA, Burrows GG, Offner H (2007) A promising therapeutic approach for multiple sclerosis: recombinant T-cell receptor ligands modulate experimental autoimmune encephalomyelitis by reducing interleukin-17 production and inhibiting migration of encephalitogenic cells into the CNS. J Neurosci 27:12531–12539. doi:10.1523/JNEUROSCI.3599-07.2007
Sinha S, Subramanian S, Emerson-Webber A, Lindner M, Burrows GG, Grafe M, Linington C, Vandenbark AA, Bernard CC, Offner H (2010) Recombinant TCR ligand reverses clinical signs and CNS damage of EAE induced by recombinant human MOG. J Neuroimmune Pharmacol 5:231–239. doi:10.1007/s11481-009-9175-1
Yadav V, Bourdette D, Bowen JD, Lynch SG, Mattson D, Preiningerova J, Rose C, Stead RB, Ferro AJ, Goldstein AS, Burrows GG, Offner H, Vandenbark AA (2010) Recombinant T cell receptor ligand (RTL) for the treatment of multiple sclerosis: report of a phase I clinical trial. Neurology 74:A293–A294
Offner H, Sinha S, Burrows GG, Ferro AJ, Vandenbark AA (2010) RTL therapy for multiple sclerosis: a phase I clinical study. J Neuroimmunol. doi:10.1016/j.jneuroim.2010.09.013
Adamus G, Burrows GG, Vandenbark AA, Offner H (2006) Treatment of autoimmune anterior uveitis with recombinant TCR ligands. Invest Ophthalmol Vis Sci 47:2555–2561. doi:10.1167/iovs.05-1242
Adamus G, Karren LJ, Mooney J, Burrows GG (2010) A promising therapeutic approach for treatment of posterior uveitis: recombinant T cell receptor ligand protects Lewis rats from acute and recurrent experimental autoimmune uveitis. Ophthalmic Res 44:24–33. doi:10.1159/000281815
Huan J, Kaler LJ, Mooney JL, Subramanian S, Hopke C, Vandenbark AA, Rosloniec EF, Burrows GG, Offner H (2008) MHC class II derived recombinant T cell receptor ligands protect DBA/1LacJ mice from collagen-induced arthritis. J Immunol 180:1249–1257
Huan J, Meza-Romero R, Mooney JL, Vandenbark AA, Offner H, Burrows GG (2010) Single-chain recombinant HLA-DQ2.5/peptide molecules block alpha2-gliadin-specific pathogenic CD4(+) T-cell proliferation and attenuate production of inflammatory cytokines: a potential therapy for celiac disease. Mucosal Immunol. doi:10.1038/mi.2010.44
Fontenot AP, Keizer TS, McCleskey M, Mack DG, Meza-Romero R, Huan J, Edwards DM, Chou YK, Vandenbark AA, Scott B, Burrows GG (2006) Recombinant HLA-DP2 binds beryllium and tolerizes beryllium-specific pathogenic CD4+ T cells. J Immunol 177:3874–3883
Subramanian S, Zhang B, Kosaka Y, Burrows GG, Grafe MR, Vandenbark AA, Hurn PD, Offner H (2009) Recombinant T cell receptor ligand treats experimental stroke. Stroke 40:2539–2545. doi:10.1161/STROKEAHA.108.543991
McCluskey J, Blok R, Kjer-Nielsen L (1989) The role of major histocompatibility molecules in antigen-specific immune responses. Transplant Proc 21:591–594
Abastado JP, Lone YC, Casrouge A, Boulot G, Kourilsky P (1995) Dimerization of soluble major histocompatibility complex-peptide complexes is sufficient for activation of T cell hybridoma and induction of unresponsiveness. J Exp Med 182:439–447
Herrmann SH, Mescher MF (1986) The requirements for antigen multivalency in class I antigen recognition and triggering of primed precursor cytolytic T lymphocytes. J Immunol 136:2816–2825
O'Herrin SM, Slansky JE, Tang Q, Markiewicz MA, Gajewski TF, Pardoll DM, Schneck JP, Bluestone JA (2001) Antigen-specific blockade of T cells in vivo using dimeric MHC peptide. J Immunol 167:2555–2560
Tsai S, Shameli A, Yamanouchi J, Clemente-Casares X, Wang J, Serra P, Yang Y, Medarova Z, Moore A, Santamaria P (2010) Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity 32:568–580. doi:10.1016/j.immuni.2010.03.015
Zuo L, Cullen CM, DeLay ML, Thornton S, Myers LK, Rosloniec EF, Boivin GP, Hirsch R (2002) A single-chain class II MHC-IgG3 fusion protein inhibits autoimmune arthritis by induction of antigen-specific hyporesponsiveness. J Immunol 168:2554–2559
Appel H, Seth NP, Gauthier L, Wucherpfennig KW (2001) Anergy induction by dimeric TCR ligands. J Immunol 166:5279–5285
Casares S, Hurtado A, McEvoy RC, Sarukhan A, von Boehmer H, Brumeanu TD (2002) Down-regulation of diabetogenic CD4+ T cells by a soluble dimeric peptide-MHC class II chimera. Nat Immunol 3:383–391. doi:10.1038/ni770ni770
Casares S, Lin M, Zhang N, Teijaro JR, Stoica C, McEvoy R, Farber DL, Bona C, Brumeanu TD (2008) A peptide-major histocompatibility complex II chimera favors survival of pancreatic beta-islets grafted in type 1 diabetic mice. Transplantation 85:1717–1725. doi:10.1097/TP.0b013e31817752cc
Preda I, McEvoy RC, Lin M, Bona CA, Rapaport R, Brumeanu TD, Casares S (2005) Soluble, dimeric HLA DR4-peptide chimeras: an approach for detection and immunoregulation of human type-1 diabetes. Eur J Immunol 35:2762–2775. doi:10.1002/eji.200526158
Lin M, Stoica-Nazarov C, Surls J, Kehl M, Bona C, Olsen C, Brumeanu TD, Casares S (2010) Reversal of type 1 diabetes by a new MHC II-peptide chimera: "Single-epitope-mediated suppression" to stabilize a polyclonal autoimmune T-cell process. Eur J Immunol 40:2277–2288. doi:10.1002/eji.200940094
Masteller EL, Warner MR, Ferlin W, Judkowski V, Wilson D, Glaichenhaus N, Bluestone JA (2003) Peptide-MHC class II dimers as therapeutics to modulate antigen-specific T cell responses in autoimmune diabetes. J Immunol 171:5587–5595
Li L, Yi Z, Wang B, Tisch R (2009) Suppression of ongoing T cell-mediated autoimmunity by peptide-MHC class II dimer vaccination. J Immunol 183:4809–4816
Lieberman S, DiLorenzo T (2003) A comprehensive guide to antibody and T-cell responses in type 1 diabetes. Tissue Antigens 62:359–377
Tsai S, Shameli A, Santamaria P (2008) CD8+ T-cells in autoimmune diabetes. Adv Immunol 100:79–124
Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, Yu L, Wegmann DR, Hutton JC, Elliott JF, Eisenbarth GS (2005) Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 435:220–223
Fan Y, Rudert WA, Grupillo M, He J, Sisino G, Trucco M (2009) Thymus-specific deletion of insulin induces autoimmune diabetes. EMBO J 28:2812–2824
Santamaria P (2010) The long and winding road to understanding and conquering type 1 diabetes. Immunity 32:437–445
Han B, Serra P, Amrani A, Yamanouchi J, Maree AF, Edelstein-Keshet L, Santamaria P (2005) Prevention of diabetes by manipulation of anti-IGRP autoimmunity: high efficiency of a low-affinity peptide. Nat Med 11:645–652. doi:10.1038/nm1250
Wang J, Tsai S, Shameli A, Yamanouchi J, Alkemade G, Santamaria P (2010) In situ recognition of autoantigen as an essential gatekeeper in autoimmune inflammation. Proc Natl Acad Sci USA 107(20):9317–9322
Lieberman S, Takaki T, Han B, Santamaria P, Serreze D, DiLorenzo T (2004) Individual nonobese diabetic mice exhibit unique patterns of CD8+ T cell reactivity to three islet antigens, including the newly identified widely expressed dystrophia myotonica kinase. J Immunol 173:6727–6734
Amrani A, Verdaguer J, Serra P, Tafuro S, Tan R, Santamaria P (2000) Progression of autoimmune diabetes driven by avidity maturation of a T-cell population. Nature 406:739–742. doi:10.1038/35021081
Aichele P, Kyburz D, Ohashi P, Odermatt B, Zinkernagel R, Hengartner H, Pircher H (1994) Peptide-induced T-cell tolerance to prevent autoimmune diabetes in a transgenic mouse model. Proc Natl Acad Sci USA 91:444–448
Toes R, Offringa R, Blom R, Melief C, Kast W (1996) Peptide vaccination can lead to enhanced tumor growth through specific T-cell tolerance induction. Proc Natl Acad Sci USA 93:7855–7860
Yamanouchi J, Verdaguer J, Han B, Amrani A, Serra P, Santamaria P (2003) Cross-priming of diabetogenic T cells dissociated from CTL-induced shedding of beta cell autoantigens. J Immunol 171:6900–6909
Jiang H, Chess L (2009) How the immune system achieves self-nonself discrimination during adaptive immunity. Adv Immunol 102:95–133
Sangkon O, Perera L, Burke D, Waldmann T, Berzofsky J (2004) IL-15/IL-15Ra-mediated avidity maturation of memory CD8+ T cells. Proc Natl Acad Sci USA 101:15154–15159
Acknowledgments
The work described here was funded by grants from the Canadian Institutes of Health Research (CIHR), the National and Engineering Research Council of Canada (NSERC), the Juvenile Diabetes Research Foundation (JDRF), the Diabetes Association (Foothills), and the Canadian Diabetes Association. X.C.C. and S.T. are supported by studentships from the AXA Research Fund Foundation and Alberta Innovates—Health Solutions (AIHS, formerly AHFMR), respectively. P.S. is a Scientist of AIHS and a JDRF Scholar. The JMDRC is supported by the Diabetes Association (Foothills).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Clemente-Casares, X., Tsai, S., Yang, Y. et al. Peptide-MHC-based nanovaccines for the treatment of autoimmunity: a “one size fits all” approach?. J Mol Med 89, 733–742 (2011). https://doi.org/10.1007/s00109-011-0757-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-011-0757-z