Abstract
The development of immunotherapies for renal cell carcinoma (RCC) has been the subject of research for several decades. In addition to cytokine therapy, the benefit of various adoptive cell therapies has again come into focus in the past several years. Nevertheless, success in fighting this immunogenic tumor is still disappointing. RCC can attract a multitude of different effector cells of both the innate and adaptive immune system, including natural killer (NK) cells, γδ T cells, NK-like T cells, peptide-specific T cells, dendritic cells (DC), and regulatory T cells (Tregs). Based on intensive research on the biology and function of different immune cells, we now understand that individual cell types do not act in isolation but function within a complex network of intercellular interactions. These interactions play a pivotal role in the efficient activation and function of effector cells, which is a prerequisite for successful tumor elimination. This review provides a current overview of the diversity of effector cells having the capacity to recognize RCC. Aspects of the functions and anti-tumor properties that make them attractive candidates for adoptive cell therapies, as well as experience in clinical application are discussed. Improved knowledge of the biology of this immune network may help us to effectively harness various effector cells, placing us in a better position to develop new therapeutic strategies to successfully fight RCC.
Similar content being viewed by others
General features of immunity to RCC
Several features of renal cell carcinoma (RCC) indicate that immune-mediated mechanisms can impinge on tumor growth and progression. Immune responses have been implicated in some cases of spontaneous tumor regression; however, experimental evidence elucidating the basis of these responses is still lacking [1]. Long-lasting regressions were rare and only some lesions disappeared in patients with multiple metastases. Several RCC patients receiving high-dose radiation of selected tumor sites showed regression of non-irradiated lesions. It was speculated that this “abscopal effect” was initiated through radiation-induced inflammation and tumor cell death, which elicited systemic immune responses that acted on distant untreated metastases [2]. Similar observations were also recorded following ultrasound treatment of RCC [3]. It remains unclear why only some tumor sites are susceptible to immune attack. Tumor cell heterogeneity may influence susceptibility to immune-mediated elimination. Alternatively, different tumor microenvironments may support or inhibit effective immune responses [4].
Clinical benefits achieved with systemic cytokine therapy provide direct evidence for development of immune responses against RCC. Remission of substantial tumor burdens was achieved in some RCC patients treated with recombinant interleukin 2 (IL-2) or interferon alpha (IFN-α) [1]. Although most responses were transient and did not affect all tumor sites, a small percentage of patients achieved long-lasting disease-free status. In fact, systemic IL-2 therapy is the only approved therapy today that provides potential cure, which is achieved in about 9% of RCC patients [5].
IL-2 mediates multiple functions that can support anti-tumor responses, including proliferation and differentiation of lymphocytes and enhancement of effector functions [6]. Recently, it was shown that the presence of IL-2 during priming of naïve T lymphocytes led to potent immunological memory [7]. Paradoxically, IL-2 can also support regulatory T cells (Tregs) that negatively impact on immune responses [8].
IFN-α can directly inhibit tumor cell proliferation, for example, through cytostatic effects or induction of apoptosis. Microarray studies of RCC cell lines displaying in vitro susceptibility or resistance to IFN-α showed no inherent differences in gene expression prior to IFN-α exposure but distinct patterns of differential gene regulation were found after stimulation. Susceptible RCC lines showed upregulation of pro-apoptotic genes whereas resistant cells had increased expression of genes with anti-apoptotic or pro-proliferation capacities [9]. IFN-α can also support both innate and adaptive immune responses directed against RCC [10]. It can directly activate natural killer (NK) cells and T cells and can induce maturational changes in dendritic cells (DC), improving their capacity to stimulate adaptive T cell responses (see Dendritic cells section). Furthermore, it can increase levels of major histocompatibility complex (MHC) class I molecules, allowing better expression of peptide-MHC (pMHC) complexes that are needed for antigen-specific T cell stimulation. Additionally, IFN-α has anti-angiogenic properties [11]. Due to their multifaceted activities, it is difficult to pinpoint the mechanisms of IL-2 and IFN-α that account for tumor regression in patients responding to systemic cytokine therapy. Therefore, prognostic factors are sought that identify potential responders in order to improve the clinical outcome of cytokine-based therapies.
In one small patient cohort, long-term survivors with stage IV disease who responded to IL-2-based immunotherapies shared a substantial number of MHC class II alleles [12]. These particular HLA molecules may allow improved presentation of RCC-associated peptides to MHC-restricted CD4+ T cells. Alternatively, as these MHC alleles are associated with increased risk for autoimmunity, tolerance to self-antigens may be less stringent in individuals with this genetic background [13].
RCC patients whose tumors expressed high levels of the carbonic anhydrase protein CA9/G250 on more than 85% of cells achieved greater clinical benefit from IL-2 therapy [14]. T cell responses to peptides derived from CA9/G250 protein have been difficult to demonstrate [15, 16], thus a role of antigen-specific immunity to G250 remains unclear. The CA9/G250 protein is involved in important intracellular signaling pathways in RCC [17]. It may thereby influence other characteristics that impact on immune-mediated recognition and elimination.
Inflammatory cells have also been proposed as a prognostic factor for clinical outcome of cytokine-based therapies. Elevated levels of circulating or tumor-infiltrating neutrophils and increased numbers of peripheral blood monocytes were correlated with decreased survival following cytokine treatment [18, 19]. How these cells impact on patient survival is not known. Neutrophils, for example, can promote angiogenesis and thereby may facilitate tumor progression [20].
The immune system: various players to fight RCC
Further insight into mechanisms of anti-tumor responses can be deduced from immunohistochemical studies of RCC tissues. Abundant cellular infiltrates, containing cells of both the innate and adaptive immune system, are often found in RCC, particularly in clear cell carcinomas (ccRCC) [1, 21]. Tumor-infiltrating lymphocytes (TIL) are rich in CD4+ and CD8+ cells and NK cells are also found in TIL of most ccRCC [22, 23]. The clinical relevance of CD8+ infiltrates was assessed in more than 200 patients who received no previous therapy [24]. It was found that an abundance of CD8+ and CD4+ T cells was associated with shorter survival and correlated with high tumor grade, suggesting that biological changes in RCC may allow the accumulation of lymphocytes. In contrast, increased proliferation of intratumoral CD8+ T cells was associated with longer patient survival. Therefore, the functional status of T cells in situ rather than the numbers of infiltrating cells may denote a better efficacy of naturally developing anti-tumor immunity.
B lymphocytes were rarely detected among RCC infiltrates. However, the SEREX method, which uses patient serum antibodies to identify tumor-associated antigens (TAA), was used successfully to identify RCC-associated proteins [25]. The presence of high-titer IgG antibodies specific for RCC-associated proteins indicates that effective cellular interactions occurred between CD4+ helper T cells and B lymphocytes in antibody-positive patients.
The complex mixtures of immune cells present in RCC mask the contribution of individual cell types to effective anti-tumor responses. Assessment of function and molecular specificity of various lymphocyte subsets therefore requires isolation and characterization of distinct cells.
Natural killer cells
NK cells express various activating and inhibitory receptors that regulate their cytokine secretion and cytotoxicity. Therefore, the capacity of NK cells to attack tumor cells depends on the balance of signals they receive from activating versus inhibitory ligands expressed by RCC [10].
Analysis of TIL revealed that NK cells were present in most RCC. Two groups of ccRCC were distinguished according to their NK infiltrate: tumors with a high percentage (>20% of TIL) or a low percentage (<20% of TIL) of NK cells. Most NK cells from tumors with high NK cell content expressed CD16 (FcγRIII receptor) and showed a higher level of intracellular cytotoxic effector molecules compared to tumors with a low NK infiltrate [26]. All NK cells from tumor tissue failed to mediate cytotoxic function directly after isolation, however NK cells from tumors with high NK percentage gained function after stimulation with IL-2 for 24 h [22, 26]. Phenotypic analysis of tumor-infiltrating NK cells revealed differences to circulating peripheral NK cells with respect to expression of killer inhibitory receptors (KIR) and C-type lectin inhibitory receptors [22].
The recruitment of NK cells might be tumor-guided, since the percentage of NK cells within the tumor did not necessarily correlate to the proportions of NK cells in the patients` PBL [22, 23]. The factors that determine the recruitment of NK cell subsets into tumors are unknown, but a role of chemokine receptors has been suggested [27]. Additionally, tumor cells and other cells, including DC, may also form specific microenvironments that impact on NK cell recruitment and function. A better understanding of the regulatory circuits and also the communication of NK cells with other immune cell populations, such as DC, is necessary to reveal how NK cells contribute to immunity to RCC. An importance of these cells can be deduced from animal models, where the cross-talk between DC and NK cells has been linked to the development of long-lasting anti-tumor immunity mediated by tumor-specific CD8+ T cells [28]. Additionally, prominent NK infiltrates have been associated with favorable prognosis in other solid tumors [29, 30], and in our patient collective no stage IV disease was observed among tumors with a high percentage of CD16+ NK infiltrates [26].
Non-MHC-restricted effector cells
Most TIL populations showed broad killing of autologous and allogeneic tumor cells after culture with low-dose IL-2 for several weeks. Tumor recognition was not MHC-dependent since class-I-negative target cells like K562 and Daudi were also killed [1]. Cytotoxic cells with similar recognition pattern were also obtained from PBMC of RCC patients and healthy individuals through in vitro culture with high-dose IL-2. Such cytotoxic cells were designated as lymphokine-activated killer (LAK) cells [31, 32]. These broadly reactive TIL and LAK cells contain CD3- NK cells and CD3+ T cells, including CD3+CD8+, CD3+CD4+, and CD3+CD4−CD8− subpopulations. All subsets showed MHC-independent cytotoxicity, but the NK fraction had the strongest killing capacity [1, 32]. Since both CD4+ and CD8+ T cells present in LAK also showed MHC-independent target cell recognition, we refer to them as NK-like T cells. NK-like T cells are placed in the category of innate immunity based on their T cell receptor (TCR) independent function.
High expression of HLA-C or HLA-E molecules protected RCC cell lines from killing by NK-like T cells. Masking of MHC class I with specific antibodies led to tumor cell lysis, indicating that NK-like T cells expressed inhibitory receptors that were triggered by direct interaction with MHC class I molecules, as is known for NK cells [32]. However, NK-like T cells appear to be regulated by distinct inhibitory receptors since they did not express any KIR or C-type lectin inhibitory receptor present on NK cells. They also apparently express distinct activating receptors since agonistic antibodies specific for activating receptors of NK cells did not trigger NK-like T cells [32, 33].
Further studies are needed to elucidate the molecular structure of these receptors and to determine whether this receptor diversity is beneficial in RCC defense. NK cells and NK-like T cells may respond to different chemokines and have different homing capacities and thereby recognize a different spectrum of tumor cells. They may also secrete different cytokines upon contact with tumor cells, potentially extending the capacity of the innate immune system to counteract RCC development and progression.
Natural killer T cells
Natural killer T (NKT) cells represent a specific subset of T lymphocytes which express surface molecules characteristic of both NK cells and T cells [34]. They express a limited TCR repertoire which they use to recognize tumor cells. In this respect, NKT cells are distinguished from NK-like T cells which express a broad repertoire of TCR but do not use their TCR for RCC recognition.
There is increased interest in the role of NKT cells in tumor immunosurveillance [35] since these cells have the ability to link innate and adaptive immunity; however little is known regarding their function in RCC.
Following TCR stimulation, NKT cells mediate a variety of effector functions, including direct killing of target cells and immediate cytokine secretion, which can provoke the subsequent activation of other effector cells such as NK cells. Activated NKT cells upregulate CD40L and can promote DC maturation via triggering of CD40. In turn, those DC can support CD4+ and CD8+ T cell responses [35, 36]. NKT cells are activated by glycolipids presented by CD1d molecules [34] and the non-physiological glycolipid α-galactosylceramide (α-GalCer) is often used as a model antigen to activate NKT cells. Physiological antigens, especially those presented by tumor cells, are largely unknown. To date, the ganglioside GD3 is the only known tumor-associated ligand for NKT cells [37], which has been detected on small-cell lung carcinomas, melanomas, and also RCC, albeit in low frequency [38–40].
Mouse models have shown that NKT cells have strong potential to inhibit tumor growth [41] and NKT cell-mediated rejection of the murine renal cell tumor Renca was demonstrated in vivo [42].
A potential prognostic relevance of NKT cells is indicated by reduced numbers and function of circulating NKT cells observed in cancer patients [43, 44]. However, data on NKT cell function is still lacking in RCC patients.
Elucidating the role of NKT cells is complicated by the existence of different NKT cell subtypes. Type I NKT cells which express Vα24Jα18 TCRα chain have been primarily analyzed to date. CD4+, CD8+, and CD4−CD8− double-negative subtypes have been identified and differences in cytokine secretion patterns as well as differences in chemokine receptor or NK receptor expression have been reported, suggesting different immune functions [45, 46]. Type II NKT cells are less restricted in their TCRαβ repertoire and, unlike type I NKT cells, they exhibit immunosuppressive functions that may hamper effective anti-tumor responses [47].
Gamma–delta T cells
Gamma–delta (γδ) T cells are also present in TIL of RCC, but at very low frequency ([48, 49], E. Nößner unpublished observation). In peripheral blood, γδ T cells account for only a small population of T cells which primarily express Vγ9Vδ2 TCR. In epithelial tissues however they can represent a dominant population and the Vδ1 subset is prevalent [50]. RCC-infiltrating γδ T cells are diverse, comprised of either prominent Vδ1 [51], Vδ2 [52] or mixed Vδ subfamilies [53]. Due to different TCR repertoires, a selective recruitment of γδ T cells into different RCC microenvironments is suggested, which may be guided by RCC-related, patient-specific antigens [54].
Recently a protein complex consisting of a structure related to the mitochondrial ATP synthase/F1-ATPase, in combination with apolipoprotein A-I (apo A-I), was found to be recognized by Vγ9Vδ2 T cells in vitro. Since F1-ATPase is expressed by several types of tumors, it was speculated that Vγ9Vδ2 T cells may sense transformed cells via F1 protein ligated to apo A-I or other tumor proteins. Whether this mechanism also functions in vivo is unknown, but RCC lines lysed by Vγ9Vδ2 T cell clones expressed an F1-related structure [55].
Phosphorylated non-peptidic bacterial metabolites are potent activators of Vγ9Vδ2 T cells. Isopentenylpyrophosphate (IPP), a eukaryotic analog of such metabolites, can be produced in high levels by some tumor cells through dysregulation of the mevalonate pathway and thereby also serve as an agonist for Vγ9Vδ2 T cells [56].
γδ T cells can kill various epithelial tumor cells, e.g. colorectal and lung cancer, as well as cells of lymphoid malignancies in vitro [57, 58], and γδ T cells isolated from TIL of RCC or derived from PBMC also showed killing of autologous and allogeneic RCC lines [51, 52, 54]. These findings, together with results from mouse experiments [58], suggest that γδ T cells may have potential in adoptive cell therapy of RCC.
MHC-restricted peptide-specific T cells
Classical MHC-restricted T cells bearing αβ TCR (i.e., cytotoxic T lymphocytes, CTL) have also been detected in some RCC patients. They were identified occasionally in TIL or were cloned from PBMC following stimulation with autologous tumor cells in vitro. CD3+CD8+ CTL killed autologous tumor but not untransformed cells. In some cases, they also recognized allogeneic tumor cells, indicating that the corresponding pMHC ligands were shared by different RCC [1, 59]. However, most CTL appeared to recognize unique pMHC ligands that were only expressed by autologous tumor cells.
It is difficult to judge how frequently MHC-restricted CTL arise in RCC patients since their presence in TIL isolates will be masked by the presence of NK-like T cells. Since there are currently no phenotypic markers that distinguish CTL and NK-like T cells, CTL must be identified at the clonal level by their MHC-restricted specificity and CD3-dependent activation.
The presence of antigen-selected MHC-restricted T cells was evaluated using TCR repertoire and CDR3 sequence analyses of TIL [1]. Dominant TCR transcripts were seldom found in RCC, perhaps due to the frequent presence of NK-like T cells displaying broad αβ TCR repertoires (D.J. Schendel unpublished observation). Better identification of selected TCR transcripts in TIL was achieved using CDR3 length analysis which can identify clonally expanded T cells [60]. Using this method, predominant αβ TCR transcripts were observed in TIL of one out of five evaluated RCC patients [61]. Importantly, this one dominant TCR sequence was expressed by a CTL clone that killed autologous tumor cells. A second study of TIL of nine RCC patients revealed highly diverse αβ TCR repertoires [62]. Although selected TCR transcripts were identified in every TIL isolate, functional anti-tumor reactivity of the expanded T cells was not demonstrated.
We analyzed TCR transcripts of TIL isolated from two RCC patients that appeared to recognize a shared pMHC ligand. The presence of highly complex families of homologous TCR sequences with shared CDR3 lengths was detected in the primary tumors of both patients, revealing that a remarkable antigen-driven selection of T cells had occurred in vivo. This analysis provided direct evidence for the natural occurrence of highly selected adaptive immune responses to RCC in some untreated patients [63].
Ligands recognized by pMHC-specific T cells
The identification of peptides recognized by MHC-restricted T cells is of great interest, particularly for the development of antigen-specific immunotherapies. Different approaches such as screening of cDNA expression libraries, elution of peptides from MHC molecules or analysis of predicted TAA epitopes have been used to identify tumor-associated T cell epitopes [64, 65].
The ligands seen by CD4+ T cells are difficult to analyze; yet, recently, a surprisingly wide variety of peptides was isolated from MHC class II molecules and found to be recognized by circulating CD4+ T cells of RCC patients. These peptides were derived, among others, from known TAA [66]. Furthermore, CD4+ T cells recognizing an epitope of the 5T4 oncofetal antigen [67], or peptides of MAGE-6 and the tyrosine kinase receptor EphA2 [68, 69] have been identified in RCC patients.
Defining ligands for CD8+ CTL has been more successful. Peptides seen by these T cells have been shown to arise through non-classical genetic mechanisms such as reverse-strand transcription [70], post-translational protein splicing [71], or translation of alternative open reading frames of normal cellular proteins [72, 73]. Furthermore, several peptides encoded by mutated proteins were identified [74, 75] and one peptide originated from the cancer-germline antigen RAGE 1 [76]. By screening predicted epitopes of CA9/G250, an HLA-A2-restricted peptide was found that was recognized by RCC-derived CTL [77]. However, with the exception of the G250 peptide, these various pMHC ligands represent epitopes that are only expressed by individual tumors.
Alternative strategies are needed to identify pMHC ligands that are shared among RCC in order to develop broadly applicable immunotherapies. This has spurred the use of “reverse immunology”, profiling tumors to identify candidate molecules that are then evaluated for their ability to elicit T cell responses. High-throughput genomics and proteomics have been applied to analysis of RCC and a number of interesting candidate molecules are emerging [10, 78, 79].
Additionally, generation of RCC-reactive CTL following stimulation of PBMC from patients or healthy donors can be helpful for pMHC-ligand identification. In this context, stimulation of PBMC with HLA class-I-matched RCC cell lines [80], peptide-pulsed antigen-presenting cells [81] or DC loaded with RCC-derived cell lysates, tumor-derived RNA, or apoptotic tumor cells [82–84] has been performed. Also, donor-derived T cells obtained from patients treated with allogeneic hematopoietic stem cell transplantation (HSCT) proved to be useful for identification of TAA of RCC [85].
Dendritic cells
DC are essential to the establishment of effective and sustained immune responses to infection and also to tumors. Some studies have addressed the presence and phenotype of DC in RCC tissue. Consistently, DC were found to be one component of the natural immune cell infiltrate [86, 87]. Yet the reports differ regarding DC frequency, describing increased [88] or similar DC numbers in tumors compared to normal kidney tissue [87]. These differing results may be explained by differing experimental methods, such as analysis of isolated cell infiltrates or enumeration in stained tumor sections. Some studies focused on a specific subtype, such as the mature and activated DC expressing CD83+ or CD83+/CMRF-44+ and found that these DC were enriched in RCC compared to non-malignant kidney tissue [87, 88]. A subset of these DC had T cell stimulatory capacity in vitro yet the majority was functionally impaired and had reduced expression of costimulatory molecules [86–88]. This was possibly attributed to immunosuppressive factors, including IL-10, TGF-β or VEGF, which are present in the RCC microenvironment and can negatively impact on DC function and development [4].
Cumulative evidence of recent years suggests that DC are a very heterogenous population with different phenotypes and functional profiles which can be shaped by organ- and compartment-specific microenvironments [89]. Considering this complexity, one must accept that our understanding of DC in RCC is far from complete. In particular, immature DC or other DC subsets have not been addressed in any detail, although they are expected to be far more prevalent than mature DC which, due to their migratory function, should exit the tumor site to enter draining lymph nodes for T cell stimulation. Resident non-mature DC may be immunogenic or, under the influence of the tumor milieu, they may be part of a regulatory network responsible for silencing anti-tumor effector lymphocyte activity. These resident DC need to be evaluated in detail, particularly with respect to functional profiles, in order to define strategies to efficiently utilize them to stimulate efficient anti-tumor responses.
Despite our incomplete understanding of DC biology in RCC, clinical benefit may be achieved through increased recruitment of DC to tumor sites, in particular if measures are taken to support activation and ameliorate inhibitory effects of the tumor milieu. An interesting candidate for this purpose is IFN-α which was recently described to induce DC maturation and activation in vitro [90]. Moreover, an increase of CD209+/CD83+ DC was found in tumor tissues of RCC patients who were treated with a cytokine cocktail composed of IFN-α, IL-2, and GM-CSF [91]. Functionally, DC conditioned with IFN-α and loaded with RCC-associated peptides had superior capacity to stimulate CD8+ type I T cell responses and a reduced potential for Treg induction in vitro compared to DC matured with a standard cytokine cocktail [92]. In addition to stimulatory cytokines, application of Toll-like receptor (TLR) agonists might also be advantageous for DC activation [93, 94].
Effector cell antagonists: regulatory T cells and myeloid-derived suppressor cells
Although a plethora of effector cells were found in TIL populations, these cells obviously failed to control RCC progression. Current research strives to understand events occurring in the tumor microenvironment that can impede successful immune control of tumor growth. In RCC, a complex network of immunosuppressive mechanisms has developed [4], also including suppressor cells that actively inhibit effector cell function.
In recent years, Tregs have received prominent attention because of their capacity to inhibit both innate and adaptive immune responses. Elevated numbers of Tregs were detected in cancer patients and their increased frequency was associated with poor survival [95, 96]. Tregs were also found to be over-represented in PBMC of RCC patients compared to healthy controls and they were also detected in TIL [97–99]. It remains controversial whether increased frequencies of Tregs are associated with poor prognosis in RCC patients. One study suggested a higher death risk for patients with elevated numbers of circulating Tregs [99], however an analysis of TIL from 170 RCC patients showed no association between numbers of Tregs and survival [100]. Rather, the presence of an intratumoral CD4+CD25+Foxp3− T cell population was significantly associated with cancer-specific death. The authors speculated that these FoxP3− T cells represented induced regulatory T cells since they expressed intracellular IL-10. Another study also found no correlation between the frequency of Tregs and clinical response of RCC patients treated with high-dose IL-2 [101].
In this context, understanding the effect of IL-2 in cancer patients is of great interest. While IL-2 is the only therapy that can produce potential cure of RCC [5], it is also essential for function and survival of Tregs [8]. Systemic IL-2 therapy increased the frequency of circulating Tregs in RCC patients [97, 101]. Treg numbers remained high in patients with progressive disease but they dropped to normal levels in patients showing clinical responses within four weeks post-therapy [97]. The mechanisms required to overcome immune suppression or to break immunological tolerance in patients responding to IL-2 remain to be determined. It is conceivable that the inhibitory capacity of Tregs is hampered in individual RCC patients. In this case, IL-2 might be able to support Treg expansion but not Treg function [102]. A concomitant expansion of anti-tumor effector cells may be able to abrogate the inhibitory effect of Tregs in patients responding to IL-2 therapy.
Myeloid-derived suppressor cells (MDSC) have also come into focus as suppressors of anti-tumor immune responses. Although known for several years, these cells have been reported in RCC only recently [103]. MDSC represent a very heterogeneous cell population, comprised of immature cells of myeloid origin in various states of differentiation. It is supposed that the tumor microenvironment conditions MDSC to acquire their suppressive phenotype. This enables them to inhibit T cell activation and function, mainly by interference with l-arginine metabolism [104]. Compared to healthy controls, increased numbers of MDSC have been reported in the PBMC of RCC patients and upregulation of either arginase-1 (ARG1) or reactive oxygen species (ROS) and NO, typical mediators of MDSC suppressive activity, was detected in vitro based on the subtype analyzed. Additionally, isolated MDSC inhibited T cell proliferation and function [103, 105].
Analysis of MDSC in RCC is only at the beginning and knowledge about the biology and function of these cells is mainly derived from mouse models. Therefore, more studies are required to determine the immunosuppressive potential of MDSC in RCC and the usage of multiple surface markers is necessary for specific and comparative characterization, due to their heterogeneity. Additionally, data on RCC-infiltrating MDSC are rare. So far, only one report described vascular endothelial growth factor receptor 1 (VEGFR1) positive MDSC in tumor tissue [106]. The impact of intratumoral MDSC on T cells would be of interest. For example, MDSC activity can result in downregulation of CD3ζ chain expression, however data are conflicting on this issue in RCC [4]. Furthermore, impact of MDSC on other effector cells such as DC, NKT or NK cells still needs to be investigated.
Data from mouse models and in vitro studies suggest that MDSC can negatively impact on cancer immunotherapies. Likewise, these studies also show that MDSC are not necessarily stuck in their immature suppressive phenotype. Different therapeutic approaches for elimination of these suppressor cells are discussed [104]. In this context, maturation of MDSC to antigen-presenting cells (APC) in vivo, by agents such as all-trans-retinoic acid (ATRA), for example, appears to be reasonable and promising [105, 107].
Applications in the clinic
Clinical benefit of non-MHC-restricted effector cells
The history of adoptive cell therapy of metastatic RCC (mRCC) spans more than three decades. LAK cells in combination with IL-2 formed the first cell therapy applied in mRCC patients [108]. The therapeutic LAK preparations were composed primarily of CD56+CD3- activated NK cells [109]. Pooled data from several trials including more than 500 RCC patients revealed an objective clinical response rate of 22%, however neither clinical response nor survival was significantly greater in patients treated with LAK cells plus IL-2 versus IL-2 alone [109, 110].
The second wave of adoptive cell therapy employed TIL, based on the assumption that these populations would represent MHC-restricted T cells with a better capacity to specifically eliminate tumor cells. Unseparated TIL, enriched CD3+CD8+ cells or combinations of CD4+ and CD8+ T cells were used in various trials. Rates of clinical response varied widely among different studies, ranging from 0–35% [109, 110]. A phase III trial comparing TIL plus IL-2 versus IL-2 alone provided no evidence for greater benefit in the presence of TIL. However, in this multi-center trial, there was a high rate of failure (41%) to obtain adequate TIL for patient application, which led to its discontinuation and left open the question of whether TIL therapy might have clinical benefit, as indicated by earlier phase I/II trials [111].
Our improved understanding of the cellular and molecular principles of immune responses against RCC now allows better insight into potential factors that may impact on patient responses to LAK versus TIL therapy. If NK cells and NK-like T cells have an important role in RCC immune defense, their activation by LAK/IL-2 therapy will have the highest benefit in patients whose RCC have disturbed MHC expression, whereas no clinical benefit would be expected in patients with tumors having normal or high MHC expression.
In an allogeneic leukocyte transfusion approach, a disparate inhibitory receptor repertoire of NK cells and NK-like T cells between recipient and donor might be beneficial, since it is unlikely that the various inhibitory receptors of the non-MHC-restricted effector cells will be matched fully by the set of MHC class I molecules on the allogeneic tumor cells, allowing the NK and NK-like T cells to escape from inhibition [32, 112]. This principle has been shown to underlie the elimination of leukemic cells in the setting of allogeneic stem cell transplantation (SCT) [113] and may also contribute to the clinical benefit of allogeneic SCT in RCC [114]. The possibility for unwanted reactions exists however if the normal tissue fails to express the necessary inhibitory ligands and additionally expresses ligands that activate non-MHC-restricted effector cells.
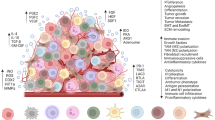
The adoptive transfer of more complex TIL infiltrates, including both MHC-restricted and non-MHC-restricted effector cells, might be advantageous. In this setting, NK cells might support adaptive T cell responses through an NK-DC axis and, in addition, they may limit the emergence of MHC class-I-negative tumor cells that cannot be eliminated by MHC-restricted CTL. That this is an important consideration can be deduced from results of immunotherapies of melanoma where induction of antigen-specific CTL often led to outgrowth of immune-escape tumor cell variants that lost pMHC ligands [115]. The capacity of NK cells to attack MHC-loss variants could therefore fulfill a special role in anti-tumor defense (Fig. 1). Nevertheless, new ways for adequate TIL expansion need to be established before these cells can be utilized for patient treatment.
The cooperation of effector cells of the innate and adaptive immune system in RCC recognition. a RCC with normal pMHC expression can inhibit NK cells through interaction of MHC with inhibitory receptors (IR) expressed by the innate immune cells. Since inhibition is dominant, activation of NK cells through ligation of activating receptors (AR) and activating ligands (AL) expressed by RCC is inhibited. However, MHC-restricted cytotoxic T cells can recognize RCC using their TCR, which leads to T cell activation and subsequent lysis of tumor cells. b RCC can escape T cell recognition in cases of disturbed MHC expression. In this case however NK cell activation and tumor cell lysis can be initiated by AR, since inhibition of NK cells is abrogated
Type I and type II interferons can modulate MHC class I expression [11]. Both classes of interferons were used in RCC immunotherapy, but only IFN-α treatment showed clinical benefit. An explanation for the clinical results might be deduced from our in vitro studies addressing the impact of IFN-α and IFN-γ on RCC recognition by various effector cells. IFN-α only modestly upregulated MHC class I and only slightly improved recognition by MHC-restricted CTL, whereas IFN-γ strongly induced MHC class I expression and strongly enhanced CTL recognition [116, 117]. RCC cells treated with IFN-α remained susceptible to killing by NK, LAK and NK-like T cells, but IFN-γ-treated RCC became fully resistant to killing by these non-MHC-restricted effector cells [32, 116, 117]. Therefore, IL-2 and IFN-α would seem better suited than IL-2 and IFN-γ to support combined innate and adaptive immune responses against RCC. This seems to reflect clinical experience with cytokine/interferon therapies.
Gamma–delta T cells for adoptive cell therapy
Thus far the cytolytic potential of γδ T cells against RCC has been primarily demonstrated in vitro. In contrast, no indication was found for clinical benefit of γδ T cells among tumor infiltrates in an analysis of more than 200 ccRCC patients. In this study, neither increased recruitment nor expansion of γδ T cells was detected in situ and percentages of γδ T cells did not correlate with prognosis or RCC-related deaths [118]. Thus, naturally occurring tumor-infiltrating γδ T cells may be inefficient in tumor defense; however, activation and expansion of γδ T cells ex vivo may generate cells capable of recognizing RCC in vivo.
Synthetic phosphoantigens or aminobisphosphonates, which cause an intracellular accumulation of IPP, are considered for activation of Vγ9Vδ2 T cell ex vivo and in vivo, in combination with low-dose IL-2 [52, 119]. Synthetic phosphoantigen in combination with low-dose IL-2 led to specific in vitro expansion of peripheral Vγ9Vδ2 T cells of RCC patients. These cells showed strong killing of autologous and allogeneic tumor cells but only low activity against normal renal cells [52, 120].
Two clinical studies utilizing γδ T cells in mRCC patients have been reported. In both cases, γδ T cells were activated and expanded ex vivo and adoptively transferred in combination with low-dose IL-2. A slowing in tumor progression was observed in three of five patients in one study [121], while six of ten patients in the second trial maintained stable disease for substantial time periods [122], indicating that γδ T cells can contribute to effective RCC immune responses.
More clinical trials enrolling higher numbers of patients are necessary to evaluate the true potential of γδ T cells, as well as possible side effects in RCC immunotherapy. In one of the two reported clinical studies, patients suffered from side effects similar to those caused by the cytokine-release syndrome following adoptive transfer of γδ T cells [122]. It remains to be elucidated whether the unnaturally high numbers of ex vivo activated T cells or concomitant activation of endogenous effector cells were responsible for an infection-like cytokine secretion. Therefore, future trials will need to better monitor the action of γδ T cells in vivo not only to assess their anti-tumor potential, but also to minimize side effects caused by unanticipated immune reactions. Additionally, the impact of different γδ T cell subsets and IL-2 needs to be considered. Furthermore, some tumor microenvironments may alter γδ T cell function. Membrane-bound MICA/B, which can be expressed by RCC [52], can activate Vδ1 and Vδ2 T cells through their TCR and NKG2D, respectively [123]. Tumor-derived soluble MICA however can down-regulate NKG2D receptors and consequently decrease or inhibit γδ T cell function [124]. Application of anti-MICA antibodies may help to retain γδ T cell activity [125]. γδ T cells can also express inhibitory NK cell receptors that control self-reactivity, thus RCC that express high levels of MHC may be protected from lysis by γδ T cells.
Developing adoptive therapies with pMHC-specific T cells
To analyze the clinical benefit of MHC-restricted peptide-specific αβ T cells, it is necessary to identify, isolate, and expand tumor-reactive CTL. This has proven to be difficult in the case of RCC since CTL may be relatively rare in RCC patients. Therefore, the development of designer T cells that are equipped with TCR specific for a defined TAA represents an attractive substitute. Several studies have analyzed the potential of lymphocytes expressing transgenic TCR (tg-TCR), for example for the treatment of hematologic malignancies [126] and melanoma [127]. Clinical studies using tg-TCR for RCC have not yet been reported.
The isolation of RCC-specific high-avidity TCR, the efficient transfer into recipient lymphocytes and subsequent TCR expression at adequate levels are crucial for the development of adoptive immunotherapy using tg-TCR T cells. We have used a model system to study the transfer of an RCC-specific TCR [63] using an optimized retrovirus to infect PBMC of healthy individuals [128]. The tg-TCR lymphocytes displayed the same specificity and killing potential as the parental TIL clone and T cell functions were long-lasting in transduced lymphocytes [129]. This demonstrated proof of concept for adoptive therapy of RCC, but identification of appropriate tg-TCR remains a major hurdle. Most MHC-restricted peptide-specific T cells recognize pMHC ligands unique to individual RCC, making their TCR sequences unsuitable for the treatment of a larger patient collective. Identification of specific TCR sequences for pMHC ligands commonly expressed by RCC is only in early stages of discovery and development.
Recently, a nonclassical CD4+ T cell clone was identified which specifically recognized a panel of RCC cell lines in a TCR-dependent but MHC-independent manner [130]. The adoptive transfer of a tg-TCR derived from such a T cell clone provides an attractive alternative to the use of classical pMHC-specific αβ TCR.
Mispairing of tg-TCR α and β chains with endogenous TCR chains remains a particular problem. Mispairing can limit expression of the tg-TCR and can lead to autoimmune reactions, if self-antigens are recognized by mispaired TCR. To minimize this risk, selection of tg-TCR sequences that preferably pair only with each other is important. In this context, the model of “strong” and “weak” TCR has been developed [131], whereby the α and β chains of a “strong” TCR preferentially pair and allow stable cell surface expression. Such “strong” tg-TCR inhibit the surface expression of “weak” endogenous TCR but they can be co-expressed on the surface with “strong” endogenous TCR. This model has been successfully demonstrated with two different tg-TCR specific for RCC [131]. Preferential expression of some tg-TCR was also observed by others [132, 133]. The characteristics responsible for stable tg-TCR pairing are not yet understood but intrinsic affinities of the α and β chains are important for chain pairing as well as for TCR assembly with CD3. This may be controlled in part by sequences of the variable regions [131, 133].
Since the number of RCC-specific TCR is currently limited, selection of tg-TCR on the basis of “weak” and “strong” is not possible yet, but alternative methods can be applied to stabilize αβ pairing [134]. Furthermore, means to specifically eliminate tg-TCR lymphocytes in vivo are also in development [135]. Therefore, adoptive transfer of tg-TCR lymphocytes may soon become available for RCC patients.
However, once tg-pMHC-specific T cells or autologous CTL have been generated for adoptive transfer, different events can limit efficiency of T cell activity in vivo. Development of antigen-loss variants of tumor cells may be one such event. Function of adoptively transferred tumor-specific T cells can also be inhibited by various tumor-derived factors, such as gangliosides or TGF-β [4]. Additionally, Tregs and MDSC may also inhibit potent anti-tumor responses. Accordingly, it was observed in an RCC mouse model that adoptively transferred high avidity CTL lost their function after tumor infiltration [136]. So called cytokine sinks may also limit pMHC-specific T cell function. In this case, cytokines needed for the proliferation, persistence and function of high numbers of transferred T cells are not available, since they are used by other endogenous cells [137]. These different hindrances may have impacted on earlier adoptive cell therapy studies with TIL or CD8+-enriched TIL that showed only limited success. Non-myeloablative lymphodepletion prior to adoptive T cell transfer appears to be a promising approach for elimination of inhibitory cells and cytokine-competing cells. Although, no clinical data are yet available for RCC, encouraging results have been achieved in the treatment of melanoma [138] and results from clinical trials using HSCT following non-myeloablative conditioning for advanced RCC patients also underscore the advantage of such an approach [139]. Nevertheless, ensuring tumor specificity of adoptively transferred T cells remains a major hurdle, especially in cases of high avidity tg-TCR CTL transferred under conditions preventing tolerizing mechanisms and in cases where the target antigen is not exclusively expressed on the tumor tissue. However, mild forms of autoimmunity may be tolerable, if tumor regression can be induced.
Allogeneic hematopoietic stem cell transplantation
Immune system control of RCC has been clearly documented in the setting of allogeneic HSCT. The first clinical trial of 19 patients with mRCC achieved an objective response rate of about 50% [140]. Other clinical trials with RCC patients have achieved response rates ranging from 0–57%, with an average of approximately 20% [114]. Since most patients had refractory tumors and very poor prognosis, these results are promising. Furthermore, HSCT has shown the most promising results in RCC compared to various other solid tumors [141].
The mechanisms leading to tumor regression have yet to be elucidated. Donor-derived NK cells can mediate anti-tumor responses in patients with hematologic malignancies following HSCT [113, 142]; however, their role in rejection of allogeneic epithelial tumors in vivo is largely unknown.
Donor-derived T cells recognizing host alloantigens have been identified as mediators of graft-versus-tumor (GVT) effects. The number of IFN-γ-producing CD8+ T cells was increased in RCC patients responding to HSCT compared to non-responding patients [143] and identification of their ligands is an important area of research.
Minor histocompatibility antigens (mHag) presented by tumor cells were shown to be crucial for development of CD8+ anti-tumor responses [144, 145]. However, expression of such mHag in normal tissues can cause graft-versus-host disease (GVHD). The identification of RCC-specific mHag and the generation of mHag-specific CTL for adoptive cell transfer would be helpful to improve GVT effects and reduce the risk of GVHD. Some RCC patients showed tumor remission without GVHD, supporting the assumption that mHag exist that are either RCC-specific or over-represented in tumor tissue [140, 146].
A CTL clone obtained from one HSCT patient, who showed a partial tumor regression without GVHD, was found to be specific for the mHag HA-1H, which was expressed by several allogeneic RCC cell lines [146]. Further studies, particularly using primary RCC tumor cells, are necessary to evaluate the potential of HA-1H in anti-tumor responses.
Several attempts have been made to identify RCC-associated TAA that can induce CTL following allogeneic HSCT. WT1 has been reported as one potential tumor antigen that may contribute to GVT response in RCC patients [147]. Recently, a CTL clone specific for a human endogenous retrovirus type E (HERV-E)-derived epitope was isolated from an RCC patient who showed tumor regression following allogeneic HSCT. This CTL clone killed HLA-A11-matched ccRCC cell lines but did not recognize non-malignant cells in vitro. Additionally, HERV-E was found to be expressed in RCC but not in normal renal tissues [85].
Clinical use of dendritic cells
One can envisage utilizing DC in two different therapeutic strategies: recruitment and activation of endogenous DC or the application of ex vivo generated DC with improved qualities.
There is limited information about how one might activate the endogenous DC population and subsequently achieve clinical benefit, but increased numbers of DC in RCC tissue following IFN-α treatment were positively correlated with patient survival [148, 149]. The factors responsible for recruitment and activation of endogenous DC are unknown but particularly in the case of IFN-α, DC might require cross-talk with NK cells to achieve full maturation [150]. An improved understanding of the tumor microenvironment and the DC subsets in RCC may shed light on this problem and may be helpful to improve cytokine therapies.
In recent years, significant effort has been directed towards the development of DC-based vaccine strategies to bypass the impaired induction of effective anti-RCC responses and generally these vaccines have been well tolerated. Yet, the clinical benefit of DC vaccines utilizing either immature or mature autologous DC, loaded with tumor lysates, tumor-associated peptides or tumor-derived RNA has been disappointing [151]. Currently, the characteristics associated with fully functional DC in vivo are unclear. Most trials employed DC generated in vitro using IL-4 and GM-CSF, without or following incubation with a cocktail containing IL-6, PGE2, TNF-α, and IL-1β [152]. Meanwhile, it is known that these DC do not secrete IL-12p70, which is essential to polarize towards Th1-type immune responses [153]. Those DC could be tolerogenic or induce Th2-polarized immune responses [154, 155].
In order to improve the DC subtype used for vaccination, we have evaluated new cytokine combinations, including TLR ligands. Monocyte-derived DC treated with these cocktails displayed phenotypic maturation and secreted high amounts of bioactive IL-12p70 [94].
In addition to cytokine secretion, appropriate antigen presentation by DC determines their capacity to prime and propagate tumor-reactive T cells. Transfection of mature DC with tumor-derived RNA provides them with a large repertoire of TAA for presentation. We found that supplying them with single-species or small pools of mRNA resulted in better antigen presentation than the use of total tumor-derived RNA. This led to the conclusion that pre-selecting a pool of defined mRNA species that encode RCC-associated antigens expressed in a majority of tumors will be advantageous for DC vaccine development [156, 157].
In addition to the priming of naïve T cells, adjuvant utilization of DC vaccines in settings of adoptive transfer of NK, NKT, and γδ T cells, may prolong effector cell function and longevity [158, 159].
Depletion of regulatory T cells
Although the precise correlation between the frequency of Tregs and clinical outcome in RCC patients is unclear, it is well accepted that Tregs can suppress the function of both innate and adaptive effector cells [160]. Therefore, depletion of Tregs prior to immunotherapy should help to improve anti-tumor responses.
Tumor-specific T cell responses were enhanced in vitro if Tregs were depleted prior to stimulation by DC presenting TAA. In these studies, either antibody-coated magnetic beads or the IL-2 diphtheria-toxin fusion protein ONTAK were used for elimination of CD25+ cells [67, 98]. Anti-CD25 magnetic beads do not discriminate between CD25high and CD25low-int expressing cells and thereby carry the risk of eliminating CD25-expressing effector T cells, while ONTAK was shown to selectively kill CD25high Tregs in vitro [98]. However, ONTAK seems not to be effective in Treg depletion in all patients [161].
The effect of Treg depletion has been analyzed in RCC patients. In one study, ten mRCC patients were given a DC-based vaccine alone or in combination with a single ONTAK treatment prior to DC application. Although the peripheral Tregs were only transiently eliminated, the Treg-depleted patients showed significantly enhanced and prolonged tumor-specific CD8+ T cell responses and also had somewhat increased numbers of specific CD4+ T cells compared to patients receiving only DC vaccine cells. Unfortunately, the clinical responses and impact on survival rate were not reported in this study [98].
In a different study, six patients with mRCC underwent leukapheresis with subsequent depletion of CD25+ T cells. Patients were lympho-depleted using non-myeloablative chemotherapy and CD25-depleted autologous cells were reinfused. Treg numbers recovered after several weeks and only one patient showed an increased T cell response to a tumor-specific antigen. Interestingly, this patient had the highest pre-treatment frequency of Treg cells [162]. In future clinical studies, analysis of larger numbers of patients is necessary to elucidate the influence of Tregs on patient survival in RCC.
The transient nature of Treg depletion remains a major problem since this might limit effective anti-tumor activity. However, how reappearance of Tregs after depletion influences clinical outcome is not yet known. Long-term elimination of Tregs may harbor various risks, such as development of severe autoimmunity. Two different methods of Treg elimination are under investigation: ex vivo depletion and in vivo targeting. The latter approach is currently more problematic since specific targeting of Tregs is not yet possible. Target molecules such as CD25, cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) or glucocorticoid-induced tumor necrosis factor receptor (GITR) are also expressed on other cell types; therefore, ONTAK, for example, can only be applied in a pre-vaccination setting since treatment during immunization can also eliminate activated effector cells. Additionally, targeting various cell subsets simultaneously may disrupt the well-balanced system of activation and inactivation of immune cells and lead to unexpected immune functions.
Therefore, new strategies are needed to obtain specific elimination of Tregs. Selective inhibition of FoxP3 expression, for example, may be a reasonable approach. FoxP3, which is considered to be the only specific marker for Tregs, is expressed intracellularly, thus new surface markers are needed to ease and standardize identification of this cell population. CD127 and CD27 are currently discussed as additional markers [163, 164], however their benefit for improving Treg identification has yet to be verified.
Concluding remarks
The statement is often made that RCC belongs to the small group of tumors that are immunogenic. Therefore, it would be expected that immunotherapies are a good option for controlling this malignancy. Nevertheless, after more than 30 years of research, the clinical outcome of RCC immunotherapy is still unsatisfactory.
Recruitment of endogenous effector cells, using cytokine therapy, adoptive transfer of LAK and TIL or application of other individual effector cell populations may not provide the immune complexity needed for successful RCC control. We now understand that a variety of cell types of the innate and adaptive immune system can contribute to RCC immunity. These different cell populations do not act in isolation but engage in cross-talk with each other, thus, stimulating or inhibiting various effector cell functions (Fig. 2). Additionally, the activity of different effector cells may vary in individual patients. Therefore, combining the actions of various cell types may be more effective in fighting RCC. This would imply concomitant adoptive transfer of different effector cell types or adoptive cell transfer combined with specific in vivo activation of effector cells. In either case, transfer/activation of immune cells of both the innate and adaptive immune system might be beneficial. However, in the end, the anti-tumor potential of different effector cells in vivo will be critically determined by the tumor microenvironment, which can inhibit or hamper effective anti-tumor responses through numerous immunosuppressive mechanisms [4]. Thus, for future immunotherapies, it will be advantageous to find means that can either select for potential responders to adoptive cell therapy or that can alter the suppressive tumor milieu in order to improve clinical outcome. Recruitment and activation of various effector cells at the tumor site may hereby already support evasion of an inhibitory milieu.
Various effector cells of both the innate and adaptive immune system have the capacity to recognize RCC. Effector cells, such as αβ T cells, NK cells, NK-like T cells, γδ T cells, NKT cells and DC are integrated in a complex network of interactions and need cross-talk with each other for optimal function. However, the success of an anti-tumor response is critically determined by RCC and its microenvironment, which have the potential to either activate or inhibit these effector cells, as do specific suppressor cells such as Treg and MDSC
In this context, for adoptive cell transfer it will be essential to generate effector cells equipped with an optimized phenotype, allowing their migration, long-term persistence and re-activation at the tumor site. Additionally, a persistent activation state, upregulation of co-stimulatory molecules or activating ligands for enhanced effector function and/or activation of other effector cell types may help to penetrate the inhibitory network, since the tumor microenvironment per se might inhibit the initial activation of effector cells. For example, activation of γδ T cells in vitro in the presence of specific cytokines could induce enhanced cytolytic function and signaling. Appropriate activation of DC in vitro, e.g., using TLR ligands, is a prerequisite for the generation of potent APC expressing Th1-related cytokines. DC used in earlier DC-based vaccine trials, showing only marginal efficiency, did not always provide such a phenotype. Likewise, antigen-specific T cells displaying an early effector state are supposed to provide a more potent anti-tumor response than strongly differentiated effector memory T cells, which might be exhausted in their cytolytic potential. While many molecular and cellular aspects of immune responses to RCC remain undefined, knowledge of RCC and effector cell biology as well as intercellular interactions of effector cells is continually improving. This places us in a better position to develop therapeutic strategies that optimally harness innate and adaptive effector cells to fight metastatic disease, providing hope for future success of immunotherapy of RCC.
References
Schendel DJ, Oberneder R, Falk CS, Jantzer P, Kressenstein S, Maget B, Hofstetter A, Riethmuller G, Nossner E (1997) Cellular and molecular analyses of major histocompatibility complex (MHC) restricted and non-MHC-restricted effector cells recognizing renal cell carcinomas: problems and perspectives for immunotherapy. J Mol Med 75:400–413
Wersall PJ, Blomgren H, Pisa P, Lax I, Kalkner KM, Svedman C (2006) Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Acta Oncol 45:493–497. doi:10.1080/02841860600604611
Sanchez-Ortiz RF, Tannir N, Ahrar K, Wood CG (2003) Spontaneous regression of pulmonary metastases from renal cell carcinoma after radio frequency ablation of primary tumor: an in situ tumor vaccine? J Urol 170:178–179. doi:10.1097/01.ju.0000070823.38336.7b
Frankenberger B, Noessner E, Schendel DJ (2007) Immune suppression in renal cell carcinoma. Semin Cancer Biol 17:330–343. doi:10.1016/j.semcancer.2007.06.004
Klapper JA, Downey SG, Smith FO, Yang JC, Hughes MS, Kammula US, Sherry RM, Royal RE, Steinberg SM, Rosenberg S (2008) High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma : a retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer 113:293–301. doi:10.1002/cncr.23552
Waldmann TA (2006) The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol 6:595–601. doi:10.1038/nri1901
Williams MA, Tyznik AJ, Bevan MJ (2006) Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature 441:890–893. doi:10.1038/nature04790
Wuest TY, Willette-Brown J, Durum SK, Hurwitz AA (2008) The influence of IL-2 family cytokines on activation and function of naturally occurring regulatory T cells. J Leukoc Biol 84:973–980. doi:10.1189/jlb.1107778
Banerjee D, Chadalavada RS, Bourdon V, Korkola JE, Motzer RJ, Chaganti RS (2006) Transcriptional program associated with IFN-alpha response of renal cell carcinoma. J Interferon Cytokine Res 26:156–170. doi:10.1089/jir.2006.26.156
Frankenberger B, Regn S, Geiger C, Noessner E, Falk CS, Pohla H, Javorovic M, Silberzahn T, Wilde S, Buchner A, Siebels M, Oberneder R, Willimsky G, Pezzutto A, Blankenstein T, Schendel DJ (2005) Cell-based vaccines for renal cell carcinoma: genetically-engineered tumor cells and monocyte-derived dendritic cells. World J Urol 23:166–174. doi:10.1007/s00345-005-0505-5
Jonasch E, Haluska FG (2001) Interferon in oncological practice: review of interferon biology, clinical applications, and toxicities. Oncologist 6:34–55
Ellerhorst JA, Hildebrand WH, Cavett JW, Fernandez-Vina MA, Hodges S, Poindexter N, Fischer H, Grimm EA (2003) Heterozygosity or homozygosity for 2 HLA class II haplotypes predict favorable outcomes for renal cell carcinoma treated with cytokine therapy. J Urol 169:2084–2088. doi:10.1097/01.ju.0000065810.80617.f4
Nepom GT, Erlich H (1991) MHC class-II molecules and autoimmunity. Annu Rev Immunol 9:493–525. doi:10.1146/annurev.iy.09.040191.002425
Bui MH, Seligson D, Han KR, Pantuck AJ, Dorey FJ, Huang Y, Horvath S, Leibovich BC, Chopra S, Liao SY, Stanbridge E, Lerman MI, Palotie A, Figlin RA, Belldegrun AS (2003) Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res 9:802–811
Bleumer I, Tiemessen DM, Oosterwijk-Wakka JC, Voller MC, De Weijer K, Mulders PF, Oosterwijk E (2007) Preliminary analysis of patients with progressive renal cell carcinoma vaccinated with CA9-peptide-pulsed mature dendritic cells. J Immunother 30:116–122. doi:10.1097/01.cji.0000211318.22902.ec
Uemura H, Fujimoto K, Tanaka M, Yoshikawa M, Hirao Y, Uejima S, Yoshikawa K, Itoh K (2006) A phase I trial of vaccination of CA9-derived peptides for HLA-A24-positive patients with cytokine-refractory metastatic renal cell carcinoma. Clin Cancer Res 12:1768–1775. doi:10.1158/1078-0432.CCR-05-2253
Dorai T, Sawczuk IS, Pastorek J, Wiernik PH, Dutcher JP (2005) The role of carbonic anhydrase IX overexpression in kidney cancer. Eur J Cancer 41:2935–2947. doi:10.1016/j.ejca.2005.09.011
Atzpodien J, Reitz M (2008) Peripheral blood neutrophils as independent immunologic predictor of response and long-term survival upon immunotherapy in metastatic renal-cell carcinoma. Cancer Biother Radiopharm 23:129–134. doi:10.1089/cbr.2007.0429
Donskov F, Hokland M, Marcussen N, Torp Madsen HH, von der Maase H (2006) Monocytes and neutrophils as 'bad guys' for the outcome of interleukin-2 with and without histamine in metastatic renal cell carcinoma—results from a randomised phase II trial. Br J Cancer 94:218–226. doi:10.1038/sj.bjc.6602937
Noonan DM, De Lerma Barbaro A, Vannini N, Mortara L, Albini A (2008) Inflammation, inflammatory cells and angiogenesis: decisions and indecisions. Cancer Metastasis Rev 27:31–40. doi:10.1007/s10555-007-9108-5
Kopecky O, Lukesova S, Vroblova V, Vokurkova D, Moravek P, Safranek H, Hlavkova D, Soucek P (2007) Phenotype analysis of tumour-infiltrating lymphocytes and lymphocytes in peripheral blood in patients with renal carcinoma. Acta Med (Hradec Kralove) 50:207–212
Schleypen JS, Von Geldern M, Weiss EH, Kotzias N, Rohrmann K, Schendel DJ, Falk CS, Pohla H (2003) Renal cell carcinoma-infiltrating natural killer cells express differential repertoires of activating and inhibitory receptors and are inhibited by specific HLA class I allotypes. Int J Cancer 106:905–912. doi:10.1002/ijc.11321
Cozar JM, Canton J, Tallada M, Concha A, Cabrera T, Garrido F, Ruiz-Cabello Osuna F (2005) Analysis of NK cells and chemokine receptors in tumor infiltrating CD4 T lymphocytes in human renal carcinomas. Cancer Immunol Immunother 54:858–866. doi:10.1007/s00262-004-0646-1
Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, Suzuki Y, Shintaku I, Nagura H, Ohtani H (2001) Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res 61:5132–5136
Sahin U, Tureci O, Schmitt H, Cochlovius B, Johannes T, Schmits R, Stenner F, Luo G, Schobert I, Pfreundschuh M (1995) Human neoplasms elicit multiple specific immune responses in the autologous host. Proc Natl Acad Sci U S A 92:11810–11813
Schleypen JS, Baur N, Kammerer R, Nelson PJ, Rohrmann K, Grone EF, Hohenfellner M, Haferkamp A, Pohla H, Schendel DJ, Falk CS, Noessner E (2006) Cytotoxic markers and frequency predict functional capacity of natural killer cells infiltrating renal cell carcinoma. Clin Cancer Res 12:718–725. doi:10.1158/1078-0432.CCR-05-0857
Robertson MJ (2002) Role of chemokines in the biology of natural killer cells. J Leukoc Biol 71:173–183
Adam C, King S, Allgeier T, Braumuller H, Luking C, Mysliwietz J, Kriegeskorte A, Busch DH, Rocken M, Mocikat R (2005) DC-NK cell cross talk as a novel CD4+ T-cell-independent pathway for antitumor CTL induction. Blood 106:338–344. doi:10.1182/blood-2004-09-3775
Villegas FR, Coca S, Villarrubia VG, Jimenez R, Chillon MJ, Jareno J, Zuil M, Callol L (2002) Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer 35:23–28
Coca S, Perez-Piqueras J, Martinez D, Colmenarejo A, Saez MA, Vallejo C, Martos JA, Moreno M (1997) The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer 79:2320–2328
Rosenberg S (1985) Lymphokine-activated killer cells: a new approach to immunotherapy of cancer. J Natl Cancer Inst 75:595–603
Falk CS, Noessner E, Weiss EH, Schendel DJ (2002) Retaliation against tumor cells showing aberrant HLA expression using lymphokine activated killer-derived T cells. Cancer Res 62:480–487
von Geldern M, Simm B, Braun M, Weiss EH, Schendel DJ, Falk CS (2006) TCR-independent cytokine stimulation induces non-MHC-restricted T cell activity and is negatively regulated by HLA class I. Eur J Immunol 36:2347–2358. doi:10.1002/eji.200535387
Bendelac A, Savage PB, Teyton L (2007) The biology of NKT cells. Annu Rev Immunol 25:297–336. doi:10.1146/annurev.immunol.25.022106.141711
Motohashi S, Nakayama T (2008) Clinical applications of natural killer T cell-based immunotherapy for cancer. Cancer Sci 99:638–645. doi:10.1111/j.1349-7006.2008.00730.x
Kronenberg M (2005) Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol 23:877–900. doi:10.1146/annurev.immunol.23.021704.115742
Wu DY, Segal NH, Sidobre S, Kronenberg M, Chapman PB (2003) Cross-presentation of disialoganglioside GD3 to natural killer T cells. J Exp Med 198:173–181. doi:10.1084/jem.20030446
Tsuchida T, Saxton RE, Morton DL, Irie RF (1989) Gangliosides of human melanoma. Cancer 63:1166–1174
Blackhall FH, Shepherd FA (2007) Small cell lung cancer and targeted therapies. Curr Opin Oncol 19:103–108. doi:10.1097/CCO.0b013e328011bec3
Biswas K, Richmond A, Rayman P, Biswas S, Thornton M, Sa G, Das T, Zhang R, Chahlavi A, Tannenbaum CS, Novick A, Bukowski R, Finke JH (2006) GM2 expression in renal cell carcinoma: potential role in tumor-induced T-cell dysfunction. Cancer Res 66:6816–6825. doi:10.1158/0008-5472.CAN-06-0250
Seino K, Motohashi S, Fujisawa T, Nakayama T, Taniguchi M (2006) Natural killer T cell-mediated antitumor immune responses and their clinical applications. Cancer Sci 97:807–812. doi:10.1111/j.1349-7006.2006.00257.x
Teng MW, Westwood JA, Darcy PK, Sharkey J, Tsuji M, Franck RW, Porcelli SA, Besra GS, Takeda K, Yagita H, Kershaw MH, Smyth MJ (2007) Combined natural killer T-cell based immunotherapy eradicates established tumors in mice. Cancer Res 67:7495–7504. doi:10.1158/0008-5472.CAN-07-0941
Giaccone G, Punt CJ, Ando Y, Ruijter R, Nishi N, Peters M, von Blomberg BM, Scheper RJ, van der Vliet HJ, van den Eertwegh AJ, Roelvink M, Beijnen J, Zwierzina H, Pinedo HM (2002) A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res 8:3702–3709
Tahir SM, Cheng O, Shaulov A, Koezuka Y, Bubley GJ, Wilson SB, Balk SP, Exley MA (2001) Loss of IFN-gamma production by invariant NK T cells in advanced cancer. J Immunol 167:4046–4050
Lee PT, Benlagha K, Teyton L, Bendelac A (2002) Distinct functional lineages of human V(alpha) 24 natural killer T cells. J Exp Med 195:637–641
Gumperz JE, Miyake S, Yamamura T, Brenner MB (2002) Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med 195:625–636
Terabe M, Berzofsky JA (2007) NKT cells in immunoregulation of tumor immunity: a new immunoregulatory axis. Trends Immunol 28:491–496. doi:10.1016/j.it.2007.05.008
Mitropoulos D, Kooi S, Rodriguez-Villanueva J, Platsoucas CD (1994) Characterization of fresh (uncultured) tumour-infiltrating lymphocytes (TIL) and TIL-derived T cell lines from patients with renal cell carcinoma. Clin Exp Immunol 97:321–327
Kowalczyk D, Skorupski W, Kwias Z, Nowak J (1997) Flow cytometric analysis of tumour-infiltrating lymphocytes in patients with renal cell carcinoma. Br J Urol 80:543–547
Moser B, Eberl M (2007) gammadelta T cells: novel initiators of adaptive immunity. Immunol Rev 215:89–102. doi:10.1111/j.1600-065X.2006.00472.x
Choudhary A, Davodeau F, Moreau A, Peyrat MA, Bonneville M, Jotereau F (1995) Selective lysis of autologous tumor cells by recurrent gamma delta tumor-infiltrating lymphocytes from renal carcinoma. J Immunol 154:3932–3940
Viey E, Fromont G, Escudier B, Morel Y, Da Rocha S, Chouaib S, Caignard A (2005) Phosphostim-activated gamma delta T cells kill autologous metastatic renal cell carcinoma. J Immunol 174:1338–1347
Olive C, Nicol D, Falk MC (1997) Characterisation of gamma delta T cells in renal cell carcinoma patients by polymerase chain reaction analysis of T cell receptor transcripts. Cancer Immunol Immunother 44:27–34
Kobayashi H, Tanaka Y, Yagi J, Toma H, Uchiyama T (2001) Gamma/delta T cells provide innate immunity against renal cell carcinoma. Cancer Immunol Immunother 50:115–124
Scotet E, Martinez LO, Grant E, Barbaras R, Jeno P, Guiraud M, Monsarrat B, Saulquin X, Maillet S, Esteve JP, Lopez F, Perret B, Collet X, Bonneville M, Champagne E (2005) Tumor recognition following Vgamma9Vdelta2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-I. Immunity 22:71–80. doi:10.1016/j.immuni.2004.11.012
Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G (2003) Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med 197:163–168
Wrobel P, Shojaei H, Schittek B, Gieseler F, Wollenberg B, Kalthoff H, Kabelitz D, Wesch D (2007) Lysis of a broad range of epithelial tumour cells by human gamma delta T cells: involvement of NKG2D ligands and T-cell receptor- versus NKG2D-dependent recognition. Scand J Immunol 66:320–328. doi:10.1111/j.1365-3083.2007.01963.x
Kabelitz D, Wesch D, Pitters E, Zoller M (2004) Potential of human gammadelta T lymphocytes for immunotherapy of cancer. Int J Cancer 112:727–732. doi:10.1002/ijc.20445
Schendel DJ, Gansbacher B, Oberneder R, Kriegmair M, Hofstetter A, Riethmuller G, Segurado OG (1993) Tumor-specific lysis of human renal cell carcinomas by tumor-infiltrating lymphocytes. I. HLA-A2-restricted recognition of autologous and allogeneic tumor lines. J Immunol 151:4209–4220
Pannetier C, Cochet M, Darche S, Casrouge A, Zoller M, Kourilsky P (1993) The sizes of the CDR3 hypervariable regions of the murine T-cell receptor beta chains vary as a function of the recombined germ-line segments. Proc Natl Acad Sci U S A 90:4319–4323
Gaudin C, Dietrich PY, Robache S, Guillard M, Escudier B, Lacombe MJ, Kumar A, Triebel F, Caignard A (1995) In vivo local expansion of clonal T cell subpopulations in renal cell carcinoma. Cancer Res 55:685–690
Puisieux I, Bain C, Merrouche Y, Malacher P, Kourilsky P, Even J, Favrot M (1996) Restriction of the T-cell repertoire in tumor-infiltrating lymphocytes from nine patients with renal-cell carcinoma. Relevance of the CDR3 length analysis for the identification of in situ clonal T-cell expansions. Int J Cancer 66:201–208. doi:10.1002/(SICI)1097-0215(19960410)66:2<201::AID-IJC11>3.0.CO;2-F
Jantzer P, Schendel DJ (1998) Human renal cell carcinoma antigen-specific CTLs: antigen-driven selection and long-term persistence in vivo. Cancer Res 58:3078–3086
Wang RF, Rosenberg SA (1999) Human tumor antigens for cancer vaccine development. Immunol Rev 170:85–100
Vissers JL, De Vries IJ, Engelen LP, Scharenborg NM, Molkenboer J, Figdor CG, Oosterwijk E, Adema GJ (2002) Renal cell carcinoma-associated antigen G250 encodes a naturally processed epitope presented by human leukocyte antigen-DR molecules to CD4(+) T lymphocytes. Int J Cancer 100:441–444. doi:10.1002/ijc.10518
Dengjel J, Nastke MD, Gouttefangeas C, Gitsioudis G, Schoor O, Altenberend F, Muller M, Kramer B, Missiou A, Sauter M, Hennenlotter J, Wernet D, Stenzl A, Rammensee HG, Klingel K, Stevanovic S (2006) Unexpected abundance of HLA class II presented peptides in primary renal cell carcinomas. Clin Cancer Res 12:4163–4170. doi:10.1158/1078-0432.CCR-05-2470
Elkord E, Burt DJ, Drijfhout JW, Hawkins RE, Stern PL (2008) CD4+ T-cell recognition of human 5T4 oncofoetal antigen: implications for initial depletion of CD25+ T cells. Cancer Immunol Immunother 57:833–847. doi:10.1007/s00262-007-0419-8
Tatsumi T, Kierstead LS, Ranieri E, Gesualdo L, Schena FP, Finke JH, Bukowski RM, Brusic V, Sidney J, Sette A, Logan TF, Kasamon YL, Slingluff CL Jr, Kirkwood JM, Storkus WJ (2003) MAGE-6 encodes HLA-DRbeta1*0401-presented epitopes recognized by CD4+ T cells from patients with melanoma or renal cell carcinoma. Clin Cancer Res 9:947–954
Tatsumi T, Herrem CJ, Olson WC, Finke JH, Bukowski RM, Kinch MS, Ranieri E, Storkus WJ (2003) Disease stage variation in CD4+ and CD8+ T-cell reactivity to the receptor tyrosine kinase EphA2 in patients with renal cell carcinoma. Cancer Res 63:4481–4489
Van Den Eynde BJ, Gaugler B, Probst-Kepper M, Michaux L, Devuyst O, Lorge F, Weynants P, Boon T (1999) A new antigen recognized by cytolytic T lymphocytes on a human kidney tumor results from reverse strand transcription. J Exp Med 190:1793–1800
Hanada K, Yewdell JW, Yang JC (2004) Immune recognition of a human renal cancer antigen through post-translational protein splicing. Nature 427:252–256. doi:10.1038/nature02240
Ronsin C, Chung-Scott V, Poullion I, Aknouche N, Gaudin C, Triebel F (1999) A non-AUG-defined alternative open reading frame of the intestinal carboxyl esterase mRNA generates an epitope recognized by renal cell carcinoma-reactive tumor-infiltrating lymphocytes in situ. J Immunol 163:483–490
Probst-Kepper M, Stroobant V, Kridel R, Gaugler B, Landry C, Brasseur F, Cosyns JP, Weynand B, Boon T, Van Den Eynde BJ (2001) An alternative open reading frame of the human macrophage colony-stimulating factor gene is independently translated and codes for an antigenic peptide of 14 amino acids recognized by tumor-infiltrating CD8 T lymphocytes. J Exp Med 193:1189–1198
Brandle D, Brasseur F, Weynants P, Boon T, Van den Eynde B (1996) A mutated HLA-A2 molecule recognized by autologous cytotoxic T lymphocytes on a human renal cell carcinoma. J Exp Med 183:2501–2508
Gaudin C, Kremer F, Angevin E, Scott V, Triebel F (1999) A hsp70–2 mutation recognized by CTL on a human renal cell carcinoma. J Immunol 162:1730–1738
Gaugler B, Brouwenstijn N, Vantomme V, Szikora JP, Van der Spek CW, Patard JJ, Boon T, Schrier P, Van den Eynde BJ (1996) A new gene coding for an antigen recognized by autologous cytolytic T lymphocytes on a human renal carcinoma. Immunogenetics 44:323–330
Vissers JL, De Vries IJ, Schreurs MW, Engelen LP, Oosterwijk E, Figdor CG, Adema GJ (1999) The renal cell carcinoma-associated antigen G250 encodes a human leukocyte antigen (HLA)-A2.1-restricted epitope recognized by cytotoxic T lymphocytes. Cancer Res 59:5554–5559
Lam JS, Pantuck AJ, Belldegrun AS, Figlin RA (2007) Protein expression profiles in renal cell carcinoma: staging, prognosis, and patient selection for clinical trials. Clin Cancer Res 13:703s–708s. doi:10.1158/1078-0432.CCR-06-1864
Stevanovic S (2002) Identification of tumour-associated T-cell epitopes for vaccine development. Nat Rev Cancer 2:514–520. doi:10.1038/nrc841
Dorrschuck A, Schmidt A, Schnurer E, Gluckmann M, Albrecht C, Wolfel C, Lennerz V, Lifke A, Di Natale C, Ranieri E, Gesualdo L, Huber C, Karas M, Wolfel T, Herr W (2004) CD8+ cytotoxic T lymphocytes isolated from allogeneic healthy donors recognize HLA class Ia/Ib-associated renal carcinoma antigens with ubiquitous or restricted tissue expression. Blood 104:2591–2599. doi:10.1182/blood-2004-02-0459
Schuster IG, Busch DH, Eppinger E, Kremmer E, Milosevic S, Hennard C, Kuttler C, Ellwart JW, Frankenberger B, Nossner E, Salat C, Bogner C, Borkhardt A, Kolb HJ, Krackhardt AM (2007) Allorestricted T cells with specificity for the FMNL1-derived peptide PP2 have potent antitumor activity against hematologic and other malignancies. Blood 110:2931–2939. doi:10.1182/blood-2006-11-058750
Kurokawa T, Oelke M, Mackensen A (2001) Induction and clonal expansion of tumor-specific cytotoxic T lymphocytes from renal cell carcinoma patients after stimulation with autologous dendritic cells loaded with tumor cells. Int J Cancer 91:749–756
Montagna D, Schiavo R, Gibelli N, Pedrazzoli P, Tonelli R, Pagani S, Assirelli E, Locatelli F, Pession A, Fregoni V, Montini E, Da Prada GA, Siena S, Maccario R (2004) Ex vivo generation and expansion of anti-tumor cytotoxic T-cell lines derived from patients or their HLA-identical sibling. Int J Cancer 110:76–86. doi:10.1002/ijc.20081
Heiser A, Maurice MA, Yancey DR, Coleman DM, Dahm P, Vieweg J (2001) Human dendritic cells transfected with renal tumor RNA stimulate polyclonal T-cell responses against antigens expressed by primary and metastatic tumors. Cancer Res 61:3388–3393
Takahashi Y, Harashima N, Kajigaya S, Yokoyama H, Cherkasova E, McCoy JP, Hanada K, Mena O, Kurlander R, Tawab A, Srinivasan R, Lundqvist A, Malinzak E, Geller N, Lerman MI, Childs RW (2008) Regression of human kidney cancer following allogeneic stem cell transplantation is associated with recognition of an HERV-E antigen by T cells. J Clin Invest 118:1099–1109. doi:10.1172/JCI34409
Schwaab T, Schned AR, Heaney JA, Cole BF, Atzpodien J, Wittke F, Ernstoff MS (1999) In vivo description of dendritic cells in human renal cell carcinoma. J Urol 162:567–573
Troy AJ, Summers KL, Davidson PJ, Atkinson CH, Hart DN (1998) Minimal recruitment and activation of dendritic cells within renal cell carcinoma. Clin Cancer Res 4:585–593
Thurnher M, Radmayr C, Ramoner R, Ebner S, Bock G, Klocker H, Romani N, Bartsch G (1996) Human renal-cell carcinoma tissue contains dendritic cells. Int J Cancer 68:1–7. doi:10.1002/(SICI)1097-0215(19960927)68:1<1::AID-IJC1>3.0.CO;2-V
Reis e Sousa C (2006) Dendritic cells in a mature age. Nat Rev Immunol 6:476–483. doi:10.1038/nri1845
Santini SM, Lapenta C, Belardelli F (2005) Type I interferons as regulators of the differentiation/activation of human dendritic cells: methods for the evaluation of IFN-induced effects. Methods Mol Med 116:167–181. doi:10.1385/1-59259-939-7:167
Verra N, de Jong D, Bex A, Batchelor D, Dellemijn T, Sein J, Nooijen W, Meinhardt W, Horenblas S, de Gast G, Vyth-Dreese F (2005) Infiltration of activated dendritic cells and T cells in renal cell carcinoma following combined cytokine immunotherapy. Eur Urol 48:527–533. doi:10.1016/j.eururo.2005.03.031
Gigante M, Mandic M, Wesa AK, Cavalcanti E, Dambrosio M, Mancini V, Battaglia M, Gesualdo L, Storkus WJ, Ranieri E (2008) Interferon-alpha (IFN-alpha)-conditioned DC preferentially stimulate type-1 and limit Treg-type in vitro T-cell responses from RCC patients. J Immunother 31:254–262. doi:10.1097/CJI.0b013e318167b023
VanOosten RL, Griffith TS (2007) Activation of tumor-specific CD8+ T Cells after intratumoral Ad5-TRAIL/CpG oligodeoxynucleotide combination therapy. Cancer Res 67:11980–11990. doi:10.1158/0008-5472.CAN-07-1526
Zobywalski A, Javorovic M, Frankenberger B, Pohla H, Kremmer E, Bigalke I, Schendel DJ (2007) Generation of clinical grade dendritic cells with capacity to produce biologically active IL-12p70. J Transl Med 5:18. doi:10.1186/1479-5876-5-18
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10:942–949. doi:10.1038/nm1093
Betts GJ, Clarke SL, Richards HE, Godkin AJ, Gallimore AM (2006) Regulating the immune response to tumours. Adv Drug Deliv Rev 58:948–961. doi:10.1016/j.addr.2006.05.006
Cesana GC, DeRaffele G, Cohen S, Moroziewicz D, Mitcham J, Stoutenburg J, Cheung K, Hesdorffer C, Kim-Schulze S, Kaufman HL (2006) Characterization of CD4+ CD25+ regulatory T cells in patients treated with high-dose interleukin-2 for metastatic melanoma or renal cell carcinoma. J Clin Oncol 24:1169–1177. doi:10.1200/JCO.2005.03.6830
Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J (2005) Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest 115:3623–3633. doi:10.1172/JCI25947
Griffiths RW, Elkord E, Gilham DE, Ramani V, Clarke N, Stern PL, Hawkins RE (2007) Frequency of regulatory T cells in renal cell carcinoma patients and investigation of correlation with survival. Cancer Immunol Immunother 56:1743–1753. doi:10.1007/s00262-007-0318-z
Siddiqui SA, Frigola X, Bonne-Annee S, Mercader M, Kuntz SM, Krambeck AE, Sengupta S, Dong H, Cheville JC, Lohse CM, Krco CJ, Webster WS, Leibovich BC, Blute ML, Knutson KL, Kwon ED (2007) Tumor-infiltrating Foxp3-CD4+ CD25+ T cells predict poor survival in renal cell carcinoma. Clin Cancer Res 13:2075–2081. doi:10.1158/1078-0432.CCR-06-2139
van der Vliet HJ, Koon HB, Yue SC, Uzunparmak B, Seery V, Gavin MA, Rudensky AY, Atkins MB, Balk SP, Exley MA (2007) Effects of the administration of high-dose interleukin-2 on immunoregulatory cell subsets in patients with advanced melanoma and renal cell cancer. Clin Cancer Res 13:2100–2108. doi:10.1158/1078-0432.CCR-06-1662
Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY (2005) A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol 6:1142–1151. doi:10.1038/ni1263
Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O'Neill A, Mier J, Ochoa AC (2005) Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res 65:3044–3048. doi:10.1158/0008-5472.CAN-04-4505
Talmadge JE (2007) Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin Cancer Res 13:5243–5248. doi:10.1158/1078-0432.CCR-07-0182
Kusmartsev S, Su Z, Heiser A, Dannull J, Eruslanov E, Kubler H, Yancey D, Dahm P, Vieweg J (2008) Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res 14:8270–8278. doi:10.1158/1078-0432.CCR-08-0165
Kusmartsev S, Eruslanov E, Kubler H, Tseng T, Sakai Y, Su Z, Kaliberov S, Heiser A, Rosser C, Dahm P, Siemann D, Vieweg J (2008) Oxidative stress regulates expression of VEGFR1 in myeloid cells: link to tumor-induced immune suppression in renal cell carcinoma. J Immunol 181:346–353
Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, Lush RM, Antonia S, Gabrilovich DI (2006) All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res 66:9299–9307. doi:10.1158/0008-5472.CAN-06-1690
Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, Matory YL, Skibber JM, Shiloni E, Vetto JT et al (1985) Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med 313:1485–1492
Hoffman DM, Gitlitz BJ, Belldegrun A, Figlin RA (2000) Adoptive cellular therapy. Semin Oncol 27:221–233
Dillman RO (2005) Lymphocyte therapy of renal cell carcinoma. Expert Rev Anticancer Ther 5:1041–1051. doi:10.1586/14737140.5.6.1041
Figlin RA, Thompson JA, Bukowski RM, Vogelzang NJ, Novick AC, Lange P, Steinberg GD, Belldegrun AS (1999) Multicenter, randomized, phase III trial of CD8(+) tumor-infiltrating lymphocytes in combination with recombinant interleukin-2 in metastatic renal cell carcinoma. J Clin Oncol 17:2521–2529
Igarashi T, Wynberg J, Srinivasan R, Becknell B, McCoy JP Jr, Takahashi Y, Suffredini DA, Linehan WM, Caligiuri MA, Childs RW (2004) Enhanced cytotoxicity of allogeneic NK cells with killer immunoglobulin-like receptor ligand incompatibility against melanoma and renal cell carcinoma cells. Blood 104:170–177. doi:10.1182/blood-2003-12-4438
Ruggeri L, Mancusi A, Burchielli E, Aversa F, Martelli MF, Velardi A (2007) Natural killer cell alloreactivity in allogeneic hematopoietic transplantation. Curr Opin Oncol 19:142–147. doi:10.1097/CCO.0b013e3280148a1a
Ueno NT, Childs RW (2007) What's past is prologue: lessons learned and the need for further development of allogeneic hematopoietic stem cell transplantation for renal cell carcinoma. Biol Blood Marrow Transplant 13:31–33. doi:10.1016/j.bbmt.2006.09.011
Restifo NP, Marincola FM, Kawakami Y, Taubenberger J, Yannelli JR, Rosenberg SA (1996) Loss of functional beta 2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. J Natl Cancer Inst 88:100–108
Schendel DJ, Falk CS, Nossner E, Maget B, Kressenstein S, Urlinger S, Tampe R, Gansbacher B (2000) Gene transfer of human interferon gamma complementary DNA into a renal cell carcinoma line enhances MHC-restricted cytotoxic T lymphocyte recognition but suppresses non-MHC-restricted effector cell activity. Gene Ther 7:950–959. doi:10.1038/sj.gt.3301187
Nößner E, Falk CS, Schendel DJ (2003) Interferon alpha and gamma differentially alter the susceptibility of tumor cells to lysis by MHC-restricted and non-MHC-restricted cytotoxic effector cells. In: Hiddemann W, Büchner T, Ritter J, Unterhalt M, Haferlach T (eds) Acute Leukemias IX. Springer, Berlin, pp 497–503
Inman BA, Frigola X, Harris KJ, Kuntz SM, Lohse CM, Leibovich BC, Kwon ED (2008) Questionable relevance of gamma delta T lymphocytes in renal cell carcinoma. J Immunol 180:3578–3584
Fiore F, Castella B, Nuschak B, Bertieri R, Mariani S, Bruno B, Pantaleoni F, Foglietta M, Boccadoro M, Massaia M (2007) Enhanced ability of dendritic cells to stimulate innate and adaptive immunity on short-term incubation with zoledronic acid. Blood 110:921–927. doi:10.1182/blood-2006-09-044321
Salot S, Laplace C, Saiagh S, Bercegeay S, Tenaud I, Cassidanius A, Romagne F, Dreno B, Tiollier J (2007) Large scale expansion of gamma 9 delta 2T lymphocytes: Innacell gamma delta cell therapy product. J Immunol Methods 326:63–75. doi:10.1016/j.jim.2007.07.010
Kobayashi H, Tanaka Y, Yagi J, Osaka Y, Nakazawa H, Uchiyama T, Minato N, Toma H (2007) Safety profile and anti-tumor effects of adoptive immunotherapy using gamma-delta T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunol Immunother 56:469–476. doi:10.1007/s00262-006-0199-6
Bennouna J, Bompas E, Neidhardt EM, Rolland F, Philip I, Galea C, Salot S, Saiagh S, Audrain M, Rimbert M, Lafaye-de Micheaux S, Tiollier J, Negrier S (2008) Phase-I study of Innacell gammadeltatrade mark, an autologous cell-therapy product highly enriched in gamma9delta2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother 57:1599–1609. doi:10.1007/s00262-008-0491-8
Kabelitz D, Wesch D, He W (2007) Perspectives of gammadelta T cells in tumor immunology. Cancer Res 67:5–8. doi:10.1158/0008-5472.CAN-06-3069
Groh V, Wu J, Yee C, Spies T (2002) Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 419:734–738. doi:10.1038/nature01112
Jinushi M, Hodi FS, Dranoff G (2006) Therapy-induced antibodies to MHC class I chain-related protein A antagonize immune suppression and stimulate antitumor cytotoxicity. Proc Natl Acad Sci U S A 103:9190–9195. doi:10.1073/pnas.0603503103
Heemskerk MH, Hoogeboom M, Hagedoorn R, Kester MG, Willemze R, Falkenburg JH (2004) Reprogramming of virus-specific T cells into leukemia-reactive T cells using T cell receptor gene transfer. J Exp Med 199:885–894. doi:10.1084/jem.20031110
Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA (2006) Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 314:126–129. doi:10.1126/science.1129003
Engels B, Cam H, Schuler T, Indraccolo S, Gladow M, Baum C, Blankenstein T, Uckert W (2003) Retroviral vectors for high-level transgene expression in T lymphocytes. Hum Gene Ther 14:1155–1168. doi:10.1089/104303403322167993
Engels B, Noessner E, Frankenberger B, Blankenstein T, Schendel DJ, Uckert W (2005) Redirecting human T lymphocytes toward renal cell carcinoma specificity by retroviral transfer of T cell receptor genes. Hum Gene Ther 16:799–810. doi:10.1089/hum.2005.16.799
Wang QJ, Hanada K, Yang JC (2008) Characterization of a novel nonclassical T cell clone with broad reactivity against human renal cell carcinomas. J Immunol 181:3769–3776
Sommermeyer D, Neudorfer J, Weinhold M, Leisegang M, Engels B, Noessner E, Heemskerk MH, Charo J, Schendel DJ, Blankenstein T, Bernhard H, Uckert W (2006) Designer T cells by T cell receptor replacement. Eur J Immunol 36:3052–3059. doi:10.1002/eji.200636539
Sant'Angelo DB, Cresswell P, Janeway CA Jr, Denzin LK (2001) Maintenance of TCR clonality in T cells expressing genes for two TCR heterodimers. Proc Natl Acad Sci U S A 98:6824–6829. doi:10.1073/pnas.121179998
Heemskerk MH, Hagedoorn RS, van der Hoorn MA, van der Veken LT, Hoogeboom M, Kester MG, Willemze R, Falkenburg JH (2007) Efficiency of T-cell receptor expression in dual-specific T cells is controlled by the intrinsic qualities of the TCR chains within the TCR-CD3 complex. Blood 109:235–243. doi:10.1182/blood-2006-03-013318
Stauss HJ, Cesco-Gaspere M, Thomas S, Hart DP, Xue SA, Holler A, Wright G, Perro M, Little AM, Pospori C, King J, Morris EC (2007) Monoclonal T-cell receptors: new reagents for cancer therapy. Mol Ther 15:1744–1750. doi:10.1038/sj.mt.6300216
Kieback E, Charo J, Sommermeyer D, Blankenstein T, Uckert W (2008) A safeguard eliminates T cell receptor gene-modified autoreactive T cells after adoptive transfer. Proc Natl Acad Sci U S A 105:623–628. doi:10.1073/pnas.0710198105
Janicki CN, Jenkinson SR, Williams NA, Morgan DJ (2008) Loss of CTL function among high-avidity tumor-specific CD8+ T cells following tumor infiltration. Cancer Res 68:2993–3000. doi:10.1158/0008-5472.CAN-07-5008
Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, Surh CD, Rosenberg SA, Restifo NP (2005) Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med 202:907–912. doi:10.1084/jem.20050732
Fang L, Lonsdorf AS, Hwang ST (2008) Immunotherapy for advanced melanoma. J Invest Dermatol 128:2596–2605. doi:10.1038/jid.2008.101
Roigas J, Johannsen M, Ringsdorf M, Massenkeil G (2006) Allogeneic stem cell transplantation for patients with metastatic renal cell carcinoma. Expert Rev Anticancer Ther 6:1449–1458. doi:10.1586/14737140.6.10.1449
Childs R, Chernoff A, Contentin N, Bahceci E, Schrump D, Leitman S, Read EJ, Tisdale J, Dunbar C, Linehan WM, Young NS, Barrett AJ (2000) Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N Engl J Med 343:750–758
Lundqvist A, Childs R (2005) Allogeneic hematopoietic cell transplantation as immunotherapy for solid tumors: current status and future directions. J Immunother 28:281–288
Savani BN, Mielke S, Adams S, Uribe M, Rezvani K, Yong AS, Zeilah J, Kurlander R, Srinivasan R, Childs R, Hensel N, Barrett AJ (2007) Rapid natural killer cell recovery determines outcome after T-cell-depleted HLA-identical stem cell transplantation in patients with myeloid leukemias but not with acute lymphoblastic leukemia. Leukemia 21:2145–2152. doi:10.1038/sj.leu.2404892
Harlin H, Artz AS, Mahowald M, Rini BI, Zimmerman T, Vogelzang NJ, Gajewski TF (2004) Clinical responses following nonmyeloablative allogeneic stem cell transplantation for renal cell carcinoma are associated with expansion of CD8+ IFN-gamma-producing T cells. Bone Marrow Transplant 33:491–497. doi:10.1038/sj.bmt.1704385
Akatsuka Y, Morishima Y, Kuzushima K, Kodera Y, Takahashi T (2007) Minor histocompatibility antigens as targets for immunotherapy using allogeneic immune reactions. Cancer Sci 98:1139–1146. doi:10.1111/j.1349-7006.2007.00521.x
Goulmy E (2006) Minor histocompatibility antigens: from transplantation problems to therapy of cancer. Hum Immunol 67:433–438. doi:10.1016/j.humimm.2006.03.012
Tykodi SS, Warren EH, Thompson JA, Riddell SR, Childs RW, Otterud BE, Leppert MF, Storb R, Sandmaier BM (2004) Allogeneic hematopoietic cell transplantation for metastatic renal cell carcinoma after nonmyeloablative conditioning: toxicity, clinical response, and immunological response to minor histocompatibility antigens. Clin Cancer Res 10:7799–7811. doi:10.1158/1078-0432.CCR-04-0072
Morita Y, Heike Y, Kawakami M, Miura O, Nakatsuka S, Ebisawa M, Mori S, Tanosaki R, Fukuda T, Kim SW, Tobinai K, Takaue Y (2006) Monitoring of WT1-specific cytotoxic T lymphocytes after allogeneic hematopoietic stem cell transplantation. Int J Cancer 119:1360–1367. doi:10.1002/ijc.21960
Kobayashi M, Suzuki K, Yashi M, Yuzawa M, Takayashiki N, Morita T (2007) Tumor infiltrating dendritic cells predict treatment response to immmunotherapy in patients with metastatic renal cell carcinoma. Anticancer Res 27:1137–1141
Hamada I, Kato M, Yamasaki T, Iwabuchi K, Watanabe T, Yamada T, Itoyama S, Ito H, Okada K (2002) Clinical effects of tumor-associated macrophages and dendritic cells on renal cell carcinoma. Anticancer Res 22:4281–4284
Tosi D, Valenti R, Cova A, Sovena G, Huber V, Pilla L, Arienti F, Belardelli F, Parmiani G, Rivoltini L (2004) Role of cross-talk between IFN-alpha-induced monocyte-derived dendritic cells and NK cells in priming CD8+ T cell responses against human tumor antigens. J Immunol 172:5363–5370
Schendel DJ (2007) Dendritic cell vaccine strategies for renal cell carcinoma. Expert Opin Biol Ther 7:221–232. doi:10.1517/14712598.7.2.221
Jonuleit H, Kuhn U, Muller G, Steinbrink K, Paragnik L, Schmitt E, Knop J, Enk AH (1997) Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol 27:3135–3142
Trinchieri G (2003) Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol 3:133–146. doi:10.1038/nri1001
Steinman RM, Hawiger D, Nussenzweig MC (2003) Tolerogenic dendritic cells. Annu Rev Immunol 21:685–711. doi:10.1146/annurev.immunol.21.120601.141040
Kaiko GE, Horvat JC, Beagley KW, Hansbro PM (2008) Immunological decision-making: how does the immune system decide to mount a helper T-cell response? Immunology 123:326–338. doi:10.1111/j.1365-2567.2007.02719.x
Javorovic M, Pohla H, Frankenberger B, Wolfel T, Schendel DJ (2005) RNA transfer by electroporation into mature dendritic cells leading to reactivation of effector-memory cytotoxic T lymphocytes: a quantitative analysis. Mol Ther 12:734–743. doi:10.1016/j.ymthe.2005.03.034
Javorovic M, Wilde S, Zobywalski A, Noessner E, Lennerz V, Wolfel T, Schendel DJ (2008) Inhibitory effect of RNA pool complexity on stimulatory capacity of RNA-pulsed dendritic cells. J Immunother 31:52–62. doi:10.1097/CJI.0b013e31815a1202
Reschner A, Hubert P, Delvenne P, Boniver J, Jacobs N (2008) Innate lymphocyte and dendritic cell cross-talk: a key factor in the regulation of the immune response. Clin Exp Immunol 152:219–226. doi:10.1111/j.1365-2249.2008.03624.x
Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, Palmer DC, Boni A, Muranski P, Yu Z, Gattinoni L, Antony PA, Rosenberg SA, Restifo NP (2007) Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest 117:2197–2204. doi:10.1172/JCI32205
Knutson KL, Disis ML, Salazar LG (2007) CD4 regulatory T cells in human cancer pathogenesis. Cancer Immunol Immunother 56:271–285. doi:10.1007/s00262-006-0194-y
Attia P, Maker AV, Haworth LR, Rogers-Freezer L, Rosenberg SA (2005) Inability of a fusion protein of IL-2 and diphtheria toxin (Denileukin Diftitox, DAB389IL-2, ONTAK) to eliminate regulatory T lymphocytes in patients with melanoma. J Immunother 28:582–592
Thistlethwaite FC, Elkord E, Griffiths RW, Burt DJ, Shablak AM, Campbell JD, Gilham DE, Austin EB, Stern PL, Hawkins RE (2008) Adoptive transfer of T(reg) depleted autologous T cells in advanced renal cell carcinoma. Cancer Immunol Immunother 57:623–634. doi:10.1007/s00262-007-0400-6
Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St Groth B (2006) Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med 203:1693–1700. doi:10.1084/jem.20060468
Duggleby RC, Shaw TN, Jarvis LB, Kaur G, Gaston JS (2007) CD27 expression discriminates between regulatory and non-regulatory cells after expansion of human peripheral blood CD4+CD25+ cells. Immunology 121:129–139. doi:10.1111/j.1365-2567.2006.02550.x
Acknowledgements
This work was supported by grants of the German Research Council (SFB455 and SFB-TR36), the Clinical Cooperation Group “Immune Monitoring” and the Helmholtz Aliance for “Immunotherapy” of Cancer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Geiger, C., Nößner, E., Frankenberger, B. et al. Harnessing innate and adaptive immunity for adoptive cell therapy of renal cell carcinoma. J Mol Med 87, 595–612 (2009). https://doi.org/10.1007/s00109-009-0455-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-009-0455-2