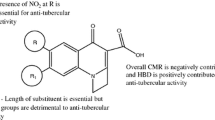
Abstract
Quantitative Structure Activity Relationship correlating the antitubercular and antibacterial (Staphylococcus aureus and Escherichia coli) activities with the structural descriptors of reported naphthyridine derivatives was developed. The data were divided into training and test sets. The former was used to develop the regression model and the latter was used to examine the predictive capability of these models. The statistical measures such as squared correlation coefficient (r 2 = 0.79–0.84), adjusted squared correlation coefficient (r 2 adj = 0.78–0.83) F-ratio (26.85–67.16), and cross-validation (q 2 = 0.74–0.79) were found to be satisfactory for all activities and the predictions were within the 99% confidence level. The models contained atom type, thermodynamic, structural, and electrotopological descriptors which emphasized the importance of the size, shape, and the lipophilicity of the molecule.



Similar content being viewed by others
Abbreviations
- QSAR:
-
quantitative structure activity relationship
- WHO:
-
World Health Organization
- SAR:
-
structure activity relationship
- MTB:
-
M. tuberculosis
- MDR-TB:
-
multi-drug resistant M. tuberculosis
- MIC:
-
minimum inhibitory concentration
- CVFF:
-
consistent valence force field
- GFA:
-
genetic function approximation technique
- LOF:
-
lack-of-fit
- LMO:
-
leave more out method
- SSE:
-
sum of squares of the error
- TSS:
-
total sum of squares
- PRESS:
-
predicted sum of squares
- ADME:
-
absorption, distribution, metabolism, and excretion
- Hf:
-
heat of formation
- Jurs-PPSA-1:
-
sum of the solvent-accessible surface areas of all positively charged atoms
References
Badawneh M, Ferrarini PL, Calderone V, Manera C, Martinotti E, Mori C, Saccomanni G, Testai L (2001) Synthesis and evaluation of antihypertensive activity of 1,8-naphthyridine derivatives. Part X. Eur J MedChem 36:925–934
Badawneh M, Manera C, Mori C, Saccomanni G, Ferrarini PL (2002) Synthesis of variously substituted 1,8-naphthyridine derivatives and evaluation of their antimycobacterial activity. Il Farmaco 57:631–639
Badawneh M, Bellini L, Cavallini T, Jamal JA, Manera C, Saccomanni G, Ferrarini PL (2003) Synthesis of 3- or 4-phenyl-1,8-naphthyridine derivatives and evaluation of antimycobacterial and antimicrobial activity. Il Farmaco 58:859–866
Bouzard D, DiCesare PP, Essiz M, Jacquet JP, Ledoussal B, Remuzon P, Kessler RE, Fung TJ (1992) Fluoronaphthyridines as antibacterial agents. 4. Synthesis and structure-activity relationships of 5-substituted-6-fluoro-7-(cycloalkylamino)-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acids. J Med Chem 35:518–525
Clark RF, Wang S, Ma Z, Weitzberg M, Motter C, Tufano M, Wagner R, Gu Y, Dandliker PJ, Lerner CG, Chovan LE, Cai Y, Black-Schaefer CL, Lynch L, Kalvin D, Nilius AM, Pratt SD, Soni N, Zhang T, Zhang X, Beutel BA (2004) Novel inhibitors of bacterial protein synthesis: structure–activity relationships for 1,8-naphthyridine derivatives incorporating position 3 and 4 variants. Bioorg Med Chem Lett 14:3299–3302
Cohen M, Huband MD, Mailloux GB, Yoder SL, Roland GE, Heifetz CL (1991) In vitro antibacterial activities of PD 131628, a new 1,8-naphthyridine anti-infective agent. Antimicrob Agents Chemother 35:141–146
Desai B, Sureja D, Naliapara Y, Shaha A, Saxena AK (2001) Synthesis and QSAR Studies of 4-Substituted phenyl-2,6-dimethyl-3,5-bis-N-(substituted phenyl)carbamoyl-1,4-dihydropyridines as potential antitubercular agents. Bioorg Med Chem 9:1993–1998
Dinakaran M, Senthilkumar P, Yogeeswari P, Sriram D (2009) Antitubercular activities of novel benzothiazolo naphthyridone carboxylic acid derivatives endowed with high activity toward multi-drug resistant tuberculosis. Biomed Pharmcother 63:11–18
Ferrarini PL, Manera C, Mori C, Badawneh M, Saccomannim G (1998) Synthesis and evaluation of antimycobacterial activity of 4-phenyl-1,8-naphthyridine derivatives. Il Farmaco 53:741–746
Ferrarini PL, Mori C, Badawneh M, Franconi F, Manera C, Miceli M, Saccomanni G (2000) Synthesis and antiplatelet activity of some 3-phenyl-1,8-naphthyridine derivatives. Il Farmaco 55:603–610
Ferrarini PL, Badawneh M, Franconi F, Manera C, Miceli M, Mori C, Saccomanni G (2001) Synthesis and antiplatelet activity of some 2,7-di(N-cycloamino)-3-phenyl-1,8-naphthyridine derivatives. Il Farmaco 56:311–318
Foroumadi A, Sakhteman A, Sharifzadeh Z, Mohammadhosseini N, Hemmateenejad B, Moshafi MH, Vosooghi M, Amini M, Shafiee A (2007) Synthesis, antituberculosis activity and QSAR study of some novel 2-(nitroaryl)-5-(nitrobenzylsulfinyl and sulfonyl)-1,3,4-thiadiazole derivatives. DARU 15(4):218–226
Ghose AK, Crippen GM (1986) Atomic physicochemical parameters for three-dimensional structure-directed quantitative structure-activity relationships I. Partition coefficients as a measure of hydrophobicity. J Comput Chem 7:565–577
Ghose AK, Viswanadhan VN, Wendoloski JJ (1998) Prediction of hydrophobic (lipophilic) properties of small organic molecules using fragmental methods: an analysis of ALOGP and CLOGP methods. J Phys Chem A 102:3762–3772
Gopalakrishnan B, Khandelwal A, Rajjak SA, Selvakumar N, Das J, Trehan S, Iqbal J, Kumar MS (2003) Three-dimensional quantitative structure–activity relationship (3D-QSAR) studies of tricyclic oxazolidinones as antibacterial agents. Bioorg Med Chem 11:2569–2574
Hopfinger AJ (1973) Conformational properties of macromolecules. Academic Press, New York
Karelson M (2000) Molecular descriptors in QSAR/QSPR. Wiley-Interscience, New York
Karki RG, Kulkarni VM (2001) Three-dimensional quantitative structure–activity relationship (3D-QSAR) of 3-aryloxazolidin-2-one antibacterials. Biorg Med Chem 9:3153–3160
Kiemele MJ, Schmidt SR, Berdine RJ (1997) Basic statistics tools for continuous improvement, vol 7, 4th edn. Air academy press Colorado springs, Colorado, p 3
Kuroda T, Suzuki F, Tamura T, Ohmori K, Hosoe H (1992) A novel synthesis and potent antiinflammatory activity of 4-hydroxy-2(1H)-oxo-1-phenyl-1,8-naphthyridine-3-carboxamides. J Med Chem 35:1130–1136
Lewin CS (1992) Antibacterial activity of a 1, 8-naphthyridine quinolone, PD 13 1628. J Med Microbiol 36:353–357
Nayyar A, Jain R (2005) Recent advances in new structural classes of anti-tuberculosis agents. Curr Med Chem 12:1873–1886
Rao GR, Mogilaiah K, Sreenivasulu B (1996) Synthesis and antimicrobial activity of 1′, 2′, 4′-triazolyl/1′, 3′, 4′-thiadiozolyl/1′, 3′, 4′-oxadiazolyl-1,8-naphthyridines and related compounds. Indian J Chem 35B:339–344
Rogers D, Hopfinger AJ (1994) Application of genetic function approximation to quantitative structure–activity relationships and quantitative structure–property relationships. J Chem Inf Comput Sci 34:854–866
Santilli A, Scotese AC, Bauer RF, Bell SC (1987) 2-Oxo-1,8-naphthyridine-3-carboxylic acid derivatives with potent gastric antisecretory properties. J Med Chem 30:2270–2277
Shagufta KumarA, Panda G, Siddiqi MI (2007) CoMFA and CoMSIA 3D-QSAR analysis of diaryloxy-methano-phenanthrene derivatives as anti-tubercular agents. J Mol Model 13:99–109
Sivakumar PM, Geetha Babu SK, Doble M (2007a) QSAR studies on chalcones and flavonoids as antituberculosis agents using genetic function approximation (GFA) method. Chem Pharm Bull 55:44–50
Sivakumar PM, Seenivasan SP, Kumar V, Doble M (2007b) Synthesis, antimycobacterial activity evaluation, and QSAR studies of chalcone derivatives. Bioorg Med Chem Lett 17:1695–1700
Sivakumar PM, Geetha Babu SK, Doble M (2008) Impact of topological and eElectronic descriptors in the QSAR of pyrazine containing thiazolines and thiazolidinones as antitubercular and antibacterial agents. Chem Biol Drug Des 71:447–463
Todeschini R, Consonni V (2000) Handbook of molecular descriptors. Wiley-VCH, Weinheim
Todeschini R, Lasagni M, Marengo E (1994) New molecular descriptors for 2D- and 3D-structures. J Chemom 8:263–273
Viswanadhan VN, Ghose AK, Reyankar GR, Robins RK (1989) Atomic physicochemical parameters for three dimensional structure directed quantitative structure-activity relationships. 4. Additional parameters for hydrophobic and dispersive interactions and their application for an automated superposition of certain naturally occurring nucleoside antibiotics. J Chem Inf Comput Sci 29:163–172
Wiese A, Munstermann M, Gutsmann T, Lindner B, Kawahara K, Zahringer U, Seydel U (1998) Molecular mechanisms of polymyxin B-membrane interactions: direct correlation between surface charge density and self-promoted transport. J Membrane Biol 162:127–138
Zhang SX, Bastow KF, Tachibana Y, Kuo SC, Hamel E, Mauger A, Narayanan VL, Lee KH (1999) Antitumor agents. 196. Substituted 2-thienyl-1,8-naphthyridin-4-ones: their synthesis, cytotoxicity, and inhibition of tubulin polymerization. J Med Chem 42:4081–4087
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sivakumar, P.M., Iyer, G. & Doble, M. QSAR studies on substituted 3- or 4-phenyl-1,8-naphthyridine derivatives as antimicrobial agents. Med Chem Res 21, 788–795 (2012). https://doi.org/10.1007/s00044-011-9564-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-011-9564-x