Abstract
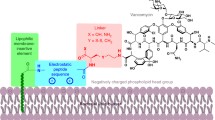
Glutamate racemase catalyses the interconversion of l-glutamate and d-glutamate making available d-glutamate which is essential for peptidoglycan biosynthesis. Inhibitors of this enzyme have exhibited antibacterial activity with the d-glutamate-analogues group of inhibitors being the most significant as it is the only group that has demonstrated efficacy in a murine thigh Streptococcus pneumoniae infection model. This group of inhibitors, however, showed a narrow antibacterial spectrum that could be due to poor lipophilicity and permeability properties. Here, we have adopted a computational ligand-based drug design approach to enhance the lipophilicity and, hence, the antibacterial spectrum of this group of inhibitors. By limiting the charged groups on our pharmacophore model and identifying key interactions for glutamate racemase binding and inhibition, we have successfully searched a compound database for compounds with both antibacterial activity and increased lipophilicity. However, our compounds appear less potent, likely due to decreased specificity. We also demonstrate that permeability and lipophilicity alone are not responsible for the narrow antibacterial spectrum observed in the d-glutamate analogue inhibitors.






Similar content being viewed by others
References
Artursson P, Karlsson J (1991) Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem Biophys Res Commun 175:880–885
Basarab GS, Hill PJ, Rastagar A, Webborn PJH (2008) Design of Helicobacter pylori glutamate racemase inhibitors as selective antibacterial agents: a novel pro-drug approach to increase exposure. Bioorg Med Chem Lett 18:4716–4722
Breault GA, Comita-Prevoir J, Eyermann CJ, Geng B, Petrichko R, Doig P, Gorseth E, Noonan B (2008) Exploring 8-benzyl pteridine-6,7-diones as inhibitors of glutamate racemase (MurI) in gram-positive bacteria. Bioorg Med Chem Lett 18:6100–6103
Chakrabarti AC (1994) Permeability of membranes to amino acids and modified amino acids: mechanisms involved in translocation. Amino Acids 6:213−229
Chakrabarti AC, Deamer DW (1992) Permeability of lipid bilayers to amino acids and phosphate. Biochim Biophys Acta 1111:171–177
Chakrabarti AC, Clark-Lewis I, Harrigan PR, Cullis PR (1992) Uptake of basic amino acids and peptides into liposomes in response to transmembrane pH gradients. Biophys J 61:228–234
Chaturvedi PR, Decker CJ, Odinecs A (2001) Prediction of pharmacokinetic properties using experimental approaches during early drug discovery. Curr Opin Chem Biol 5:452–463
De Dios A, Prieto L, Martin JA, Rubio A, Ezquerra J, Tebbe M, Lopez de Ural de B, Martin J, Sanchez A, LeTourneau DL, McGee JE, Boylan C, Parr TR, Smith MC (2002) 4-Substituted d-glutamic acid analogues: the first potent inhibitors of glutamate racemase (MurI) enzyme with antibacterial activity. J Med Chem 45:4559–4570
Doublet P, van Heijenoort J, Mengin-Lecreulx D (1992) Identification of the Escherichia coli murI gene, which is required for the biosynthesis of d-glutamic acid, a specific component of bacterial peptidoglycan. J Bacteriol 174:5772–5779
Fisher SL (2008) Glutamate racemase as a target for drug discovery. Microb Biotechnol 1:345–360
Gallo KA, Knowles JR (1993) Purification, cloning, and cofactor independence of glutamate racemase from lactobacillus. Biochemistry 32:3981–3990
Ge M, Chen Z, Onishi HR, Kohler J, Silver LL, Kerns R, Fukuzawa S, Thompson C, Kahne D (1999) Vancomycin derivatives that inhibit peptidoglycan biosynthesis without binding d-ala-d-ala. Science 284:507–511
Geng B, Breault G, Comita-Prevoir J, Petrichko R, Eyermann C, Lundqvist T, Doig P, Gorseth E, Noonan B (2008) Exploring 9-benzyl purines as inhibitors of glutamate racemase (MurI) in gram-positive bacteria. Bioorg Med Chem Lett 18:4368–4372
Geng B, Basarab G, Comita-Prevoir J, Gowravaram M, Hill P, Kiely A, Loch J, MacPherson L, Morningstar M, Mullen G, Osimboni E, Satz A, Eyermann C, Lundqvist T (2009) Potent and selective inhibitors of Helicobacter pylori glutamate racemase (MurI): pyridodiazepine amines. Bioorg Med Chem Lett 19:930–936
Glavas S, Tanner ME (1999) Catalytic acid/base residues of glutamate racemase. Biochemistry 38:4106–4113
Gutknecht J, Walter A (1981) Histamine, theophylline and tryptamine transport through lipid bilayer membranes. Biochim Biophys Acta 649:149–154
Kerns EH, Di L (2008a) Permeability. In: Drug-like properties: concepts, structure design and methods: from ADME to toxicity optimization. Academic Press, Amsterdam, Boston, pp 86–98
Kerns EH, Di L (2008b) Lipophilicity. In: Drug-like properties: concepts, structure design and methods: from ADME to toxicity optimization. Academic Press, Amsterdam, Boston, pp 43–47
Kim WC, Rhee HI, Park BK, Suk KH, Cha SH (2000) Isolation of peptide ligands that inhibit glutamate racemase activity from a random phage display library. J Biomol Screen 5:435–540
MAESTRO, version 8.5. Schrodinger, LLC, New York, NY, 2008
Miller EL (2002) The penicillins: a review and update. J Midwifery Women Health 47:426–434
PHASE, version 2.0. Schrodinger, LLC, New York, NY, 2005
QIKPROP, version 3.1 and user manual. Schrodinger, LLC, New York, NY, 2008
Seymour RA, Hogg SD (2008) Antibiotics and chemoprophylaxis. Periodontol 2000 46:80–108
Stenberg P, Luthman K, Artursson P (2000) Virtual screening of intestinal drug permeability. J Control Release 65:231–243
Tanner ME, Glavas S (1997) The inhibition of glutamate racemase by d-N-hydroxyglutamate. Bioorg Med Chem Lett 7:2265–2270
Tanner ME, Miao S (1994) The synthesis and stability of aziridino-glutamate, an irreversible inhibitor of glutamate racemase. Tetrahedron Lett 35:4073–4076
Vollmer W (2008) Peptidoglycan structure and architecture. FEMS Microbiol Rev 32:149–167
Vollmer W, Bertsche U (2008) Murein (peptidoglycan) structure, architecture and biosynthesis in Escherichia coli. Biochim Biophys Acta 1778:1714–1734
Walsh CT (1989) Enzymes in the d-alanine branch of bacterial cell wall peptidoglycan assembly. J Biol Chem 264:2393–2396
Young KD (2003) Bacterial shape. Mol Microbiol 49:571–580
Acknowledgments
We thank Dr. Linda Young, Professor of Biological Sciences, Department of Biology, Ohio Northern University, for the valuable input and discussion and for providing the S. aureus and E. coli strains used in this study. This work was supported by a Bower, Bennet, and Bennet grant from Ohio Northern University, Raabe College of Pharmacy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Skidmore, K.W., Scherer, C., Stockert, A. et al. A ligand-based approach for enhancing the pharmacokinetic profile of highly charged antibacterial agents. Med Chem Res 21, 362–372 (2012). https://doi.org/10.1007/s00044-010-9538-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-010-9538-4