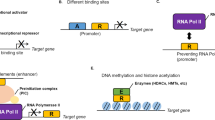
Abstract
By being the “integration” center of transcriptional control as they move and target transcription factors, corepressors fine-tune the epigenetic status of the nucleus. Many of them utilize enzymatic activities to modulate chromatin through histone modification or chromatin remodeling. The clinical and etiological relevance of the corepressors to neoplastic growth is increasingly being recognized. Aberrant expression or function (both loss and gain of) of corepressors has been associated with malignancy and contribute to the generation of transcriptional “inflexibility” manifested as distorted signaling along certain axes. Understanding and predicting the consequences of corepressor alterations in tumor cells has diagnostic and prognostic value, and also have the capacity to be targeted through selective epigenetic regimens. Here, we evaluate corepressors with the most promising therapeutic potential based on their physiological roles and involvement in malignant development, and also highlight areas that can be exploited for molecular targeting of a large proportion of clinical cancers and their complications.

Similar content being viewed by others
References
Grivas PD, Kiaris H, Papavassiliou AG (2011) Tackling transcription factors: challenges in antitumor therapy. Trends Mol Med 17:537–538
Lonard DM, O’Malley BW (2007) Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation. Mol Cell 27:691–700
Rosenfeld MG, Lunyak VV, Glass CK (2006) Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev 20:1405–1428
Payankaulam S, Li LM, Arnosti DN (2010) Transcriptional repression: conserved and evolved features. Curr Biol 20:R764–R771
Perissi V, Jepsen K, Glass CK, Rosenfeld MG (2010) Deconstructing repression: evolving models of co-repressor action. Nat Rev Gen 11:109–123
Stewart MD, Wong J (2009) Nuclear receptor repression: regulatory mechanisms and physiological implications. Prog Mol Biol Transl Sci 87:235–259
Lonard DM, Lanz RB, O’Malley BW (2007) Nuclear receptor coregulators and human disease. Endocr Rev 28:575–587
Wang Z, Zang C, Cui K, Schones DE, Barski A, Peng W, Zhao K (2009) Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell 138:1019–1031
Gurevich I, Flores AM, Aneskievich BJ (2007) Corepressors of agonist-bound nuclear receptors. Toxicol Appl Pharmacol 223:288–298
Watson PJ, Fairall L, Schwabe JW (2012) Nuclear hormone receptor co-repressors: structure and function. Mol Cell Endocrinol 348:440–449
Battaglia S, Maguire O, Campbell MJ (2010) Transcription factor co-repressors in cancer biology: roles and targeting. Int J Cancer 126:2511–2519
Biancotto C, Frige G, Minucci S (2010) Histone modification therapy of cancer. Adv Genet 70:341–386
Liu Y, Chen W, Gaudet J, Cheney MD, Roudaia L, Cierpicki T, Klet RC, Hartman K, Laue TM, Speck NA, Bushweller JH (2007) Structural basis for recognition of SMRT/N-CoR by the MYND domain and its contribution to AML1/ETO’s activity. Cancer Cell 11:483–497
Abedin SA, Banwell CM, Colston KW, Carlberg C, Campbell MJ (2006) Epigenetic corruption of VDR signalling in malignancy. Anticancer Res 26:2557–2566
Abedin SA, Thorne JL, Battaglia S, Maguire O, Hornung LB, Doherty AP, Mills IG, Campbell MJ (2009) Elevated NCOR1 disrupts a network of dietary-sensing nuclear receptors in bladder cancer cells. Carcinogenesis 30:449–456
Battaglia S, Maguire O, Thorne JL, Hornung LB, Doig CL, Liu S, Sucheston LE, Bianchi A, Khanim FL, Gommersall LM, Coulter HS, Rakha S, Giddings I, O’Neill LP, Cooper CS, McCabe CJ, Bunce CM, Campbell MJ (2010) Elevated NCOR1 disrupts PPARalpha/gamma signaling in prostate cancer and forms a targetable epigenetic lesion. Carcinogenesis 31:1650–1660
Mann M, Cortez V, Vadlamudi RK (2011) Epigenetics of estrogen receptor signaling: role in hormonal cancer progression and therapy. Cancers 3:1691–1707
Konduri SD, Medisetty R, Liu W, Kaipparettu BA, Srivastava P, Brauch H, Fritz P, Swetzig WM, Gardner AE, Khan SA, Das GM (2010) Mechanisms of estrogen receptor antagonism toward p53 and its implications in breast cancer therapeutic response and stem cell regulation. Proc Natl Acad Sci USA 107:15081–15086
Hong W, Chen L, Li J, Yao Z (2010) Inhibition of MAP kinase promotes the recruitment of corepressor SMRT by tamoxifen-bound estrogen receptor alpha and potentiates tamoxifen action in MCF-7 cells. Biochem Biophys Res Commun 396:299–303
Reeb CA, Gerlach C, Heinssmann M, Prade I, Ceraline J, Roediger J, Roell D, Baniahmad A (2011) A designed cell-permeable aptamer-based corepressor peptide is highly specific for the androgen receptor and inhibits prostate cancer cell growth in a vector-free mode. Endocrinology 152:2174–2183
Chmelar R, Buchanan G, Need EF, Tilley W, Greenberg NM (2007) Androgen receptor coregulators and their involvement in the development and progression of prostate cancer. Int J Cancer 120:719–733
van de Wijngaart DJ, Dubbink HJ, van Royen ME, Trapman J, Jenster G (2012) Androgen receptor coregulators: recruitment via the coactivator binding groove. Mol Cell Endocrinol 352:57–69
Eisold M, Asim M, Eskelinen H, Linke T, Baniahmad A (2009) Inhibition of MAPK-signaling pathway promotes the interaction of the corepressor SMRT with the human androgen receptor and mediates repression of prostate cancer cell growth in the presence of antiandrogens. J Mol Endocrinol 42:429–435
Buchanan G, Need EF, Barrett JM, Bianco-Miotto T, Thompson VC, Butler LM, Marshall VR, Tilley WD, Coetzee GA (2011) Corepressor effect on androgen receptor activity varies with the length of the CAG encoded polyglutamine repeat and is dependent on receptor/corepressor ratio in prostate cancer cells. Mol Cell Endocrinol 342:20–31
Campos B, Bermejo JL, Han L, Felsberg J, Ahmadi R, Grabe N, Reifenberger G, Unterberg A, Herold-Mende C (2011) Expression of nuclear receptor corepressors and class I histone deacetylases in astrocytic gliomas. Cancer Sci 102:387–392
Hsia EY, Goodson ML, Zou JX, Privalsky ML, Chen HW (2010) Nuclear receptor coregulators as a new paradigm for therapeutic targeting. Adv Drug Deliv Rev 62:1227–1237
Lakowski B, Roelens I, Jacob S (2006) CoREST-like complexes regulate chromatin modification and neuronal gene expression. J Mol Neurosci 29:227–239
Lim S, Janzer A, Becker A, Zimmer A, Schule R, Buettner R, Kirfel J (2010) Lysine-specific demethylase 1 (LSD1) is highly expressed in ER-negative breast cancers and a biomarker predicting aggressive biology. Carcinogenesis 31:512–520
Schulte JH, Lim S, Schramm A, Friedrichs N, Koster J, Versteeg R, Ora I, Pajtler K, Klein-Hitpass L, Kuhfittig-Kulle S, Metzger E, Schule R, Eggert A, Buettner R, Kirfel J (2009) Lysine-specific demethylase 1 is strongly expressed in poorly differentiated neuroblastoma: implications for therapy. Cancer Res 69:2065–2071
Kahl P, Gullotti L, Heukamp LC, Wolf S, Friedrichs N, Vorreuther R, Solleder G, Bastian PJ, Ellinger J, Metzger E, Schule R, Buettner R (2006) Androgen receptor coactivators lysine-specific histone demethylase 1 and four and a half LIM domain protein 2 predict risk of prostate cancer recurrence. Cancer Res 66:11341–11347
Huang Y, Stewart TM, Wu Y, Baylin SB, Marton LJ, Perkins B, Jones RJ, Woster PM, Casero RA Jr (2009) Novel oligoamine analogues inhibit lysine-specific demethylase 1 and induce reexpression of epigenetically silenced genes. Clin Cancer Res 15:7217–7228
Thillainadesan G, Isovic M, Loney E, Andrews J, Tini M, Torchia J (2008) Genome analysis identifies the p15ink4b tumor suppressor as a direct target of the ZNF217/CoREST complex. Mol Cell Biol 28:6066–6077
Straza MW, Paliwal S, Kovi RC, Rajeshkumar B, Trenh P, Parker D, Whalen GF, Lyle S, Schiffer CA, Grossman SR (2010) Therapeutic targeting of C-terminal binding protein in human cancer. Cell Cycle 9:3740–3750
Chinnadurai G (2009) The transcriptional corepressor CtBP: a foe of multiple tumor suppressors. Cancer Res 69:731–734
Zhao LZ, Chinnadurai G (2010) Incapacitating CtBP to kill cancer. Cell Cycle 9:3645–3646
Deng Y, Liu J, Han G, Lu SL, Wang SY, Malkoski S, Tan AC, Deng C, Wang XJ, Zhang Q (2010) Redox-dependent Brca1 transcriptional regulation by an NADH-sensor CtBP1. Oncogene 29:6603–6608
Grzenda A, Lomberk G, Zhang JS, Urrutia R (2009) Sin3: master scaffold and transcriptional corepressor. Biochim Biophys Acta 1789:443–450
Silverstein RA, Ekwall K (2005) Sin3: a flexible regulator of global gene expression and genome stability. Curr Genet 47:1–17
Ellison-Zelski SJ, Alarid ET (2010) Maximum growth and survival of estrogen receptor-alpha positive breast cancer cells requires the Sin3A transcriptional repressor. Mol Cancer 9:263
Farias EF, Petrie K, Leibovitch B, Murtagh J, Chornet MB, Schenk T, Zelent A, Waxman S (2010) Interference with Sin3 function induces epigenetic reprogramming and differentiation in breast cancer cells. Proc Natl Acad Sci USA 107:11811–11816
Hurst DR, Welch DR (2011) Unraveling the enigmatic complexities of BRMS1-mediated metastasis suppression. FEBS Lett 585:3185–3190
Smith KT, Martin-Brown SA, Florens L, Washburn MP, Workman JL (2010) Deacetylase inhibitors dissociate the histone-targeting ING2 subunit from the Sin3 complex. Chem Biol 17:65–74
Reisman D, Glaros S, Thompson EA (2009) The SWI/SNF complex and cancer. Oncogene 28:1653–1668
Hargreaves DC, Crabtree GR (2011) ATP-dependent chromatin remodeling: genetics, genomics and mechanisms. Cell Res 21:396–420
Wilson BG, Roberts CW (2011) SWI/SNF nucleosome remodellers and cancer. Nat Rev Cancer 11:481–492
Guan B, Wang TL, Shih IeM (2011) ARID1A, a factor that promotes formation of SWI/SNF-mediated chromatin remodeling, is a tumor suppressor in gynecologic cancers. Cancer Res 71:6718–6727
Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, Senz J, McConechy MK, Anglesio MS, Kalloger SE, Yang W, Heravi-Moussavi A, Giuliany R, Chow C, Fee J, Zayed A, Prentice L, Melnyk N, Turashvili G, Delaney AD, Madore J, Yip S, McPherson AW, Ha G, Bell L, Fereday S, Tam A, Galletta L, Tonin PN, Provencher D, Miller D, Jones SJ, Moore RA, Morin GB, Oloumi A, Boyd N, Aparicio SA, Shih IeM, Mes-Masson AM, Bowtell DD, Hirst M, Gilks B, Marra MA, Huntsman DG (2010) ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med 363:1532–1543
Wang X, Sansam CG, Thom CS, Metzger D, Evans JA, Nguyen PT, Roberts CW (2009) Oncogenesis caused by loss of the SNF5 tumor suppressor is dependent on activity of BRG1, the ATPase of the SWI/SNF chromatin remodeling complex. Cancer Res 69:8094–8101
Lai AY, Wade PA (2011) Cancer biology and NuRD: a multifaceted chromatin remodelling complex. Nat Rev Cancer 11:588–596
Manavathi B, Singh K, Kumar R (2007) MTA family of coregulators in nuclear receptor biology and pathology. Nucl Recept Signal 5:e010
Ramirez J, Hagman J (2009) The Mi-2/NuRD complex: a critical epigenetic regulator of hematopoietic development, differentiation and cancer. Epigenetics 4:532–536
Wang Y, Zhang H, Chen Y, Sun Y, Yang F, Yu W, Liang J, Sun L, Yang X, Shi L, Li R, Li Y, Zhang Y, Li Q, Yi X, Shang Y (2009) LSD1 is a subunit of the NuRD complex and targets the metastasis programs in breast cancer. Cell 138:660–672
Kai L, Samuel SK, Levenson AS (2010) Resveratrol enhances p53 acetylation and apoptosis in prostate cancer by inhibiting MTA1/NuRD complex. Int J Cancer 126:1538–1548
Asim M, Hafeez BB, Siddiqui IA, Gerlach C, Patz M, Mukhtar H, Baniahmad A (2011) Ligand-dependent corepressor acts as a novel androgen receptor corepressor, inhibits prostate cancer growth, and is functionally inactivated by the Src protein kinase. J Biol Chem 286:37108–37117
Cheng YH, Utsunomiya H, Pavone ME, Yin P, Bulun SE (2011) Retinoic acid inhibits endometrial cancer cell growth via multiple genomic mechanisms. J Mol Endocrinol 46:139–153
Richly H, Aloia L, Di Croce L (2011) Roles of the Polycomb group proteins in stem cells and cancer. Cell Death Dis 2:e204
Jennings BH, Ish-Horowicz D (2008) The Groucho/TLE/Grg family of transcriptional co-repressors. Genome Biol 9:205
Papaioannou M, Melle C, Baniahmad A (2007) The coregulator alien. Nucl Recept Signal 5:e008
Graham JS, Kaye SB, Brown R (2009) The promises and pitfalls of epigenetic therapies in solid tumours. Eur J Cancer 45:1129–1136
Cerchietti LC, Ghetu AF, Zhu X, Da Silva GF, Zhong S, Matthews M, Bunting KL, Polo JM, Farès C, Arrowsmith CH, Yang SN, Garcia M, Coop A, Mackerell AD Jr, Privé GG, Melnick A (2010) A small-molecule inhibitor of BCL6 kills DLBCL cells in vitro and in vivo. Cancer Cell 17:400–411
Baylin SB, Jones PA (2011) A decade of exploring the cancer epigenome - biological and translational implications. Nat Rev Cancer 11:726–734
Dworkin AM, Huang TH, Toland AE (2009) Epigenetic alterations in the breast: implications for breast cancer detection, prognosis and treatment. Semin Cancer Biol 19:165–171
Bantscheff M, Hopf C, Savitski MM, Dittmann A, Grandi P, Michon AM, Schlegl J, Abraham Y, Becher I, Bergamini G, Boesche M, Delling M, Dumpelfeld B, Eberhard D, Huthmacher C, Mathieson T, Poeckel D, Reader V, Strunk K, Sweetman G, Kruse U, Neubauer G, Ramsden NG, Drewes G (2011) Chemoproteomics profiling of HDAC inhibitors reveals selective targeting of HDAC complexes. Nat Biotechnol 29:255–265
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vaiopoulos, A.G., Kostakis, I.D., Athanasoula, K.C. et al. Targeting transcription factor corepressors in tumor cells. Cell. Mol. Life Sci. 69, 1745–1753 (2012). https://doi.org/10.1007/s00018-012-0986-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-0986-5