Abstract.
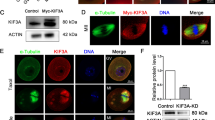
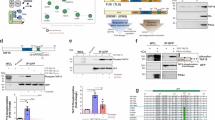
Polo-like kinase 1 (Plk1) is a highly conserved serine/threonine kinase that plays critical roles in many cell cycle events, especially in mitosis. In the present study, we identified TTDN1 as a potential interacting partner of Plk1 in yeast two-hybrid screens. Sequence analysis indicates that TTDN1 contains a consensus Plk1-binding motif at its C terminus. TTDN1 colocalizes with Plk1 at the centrosome in mitosis and the midbody during cytokinesis. TTDN1 is phosphorylated by Cdk1 in mitosis, and this is required for its interaction with Plk1. Site-directed mutagenesis indicates that TTDN1 is phosphorylated at multiple residues, including Ser93 and Ser104. Mutation of Thr120 of TTDN1 abolishes its interaction with Plk1, suggesting phosphorylation of Thr120 in the consensus Plk1-binding motif is required for its interaction with Plk1. Overexpression of TTDN1 or its knockdown by siRNA causes multi-polar spindles and multiple nuclei, suggesting that TTDN1 plays a role in regulating mitosis and cytokinesis.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Additional information
Received 27 November 2006; received after revision 4 January 2007; accepted 25 January 2007
Y. Zhang, Y. Tian: These authors contribute equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, Y., Tian, Y., Chen, Q. et al. TTDN1 is a Plk1-interacting protein involved in maintenance of cell cycle integrity. Cell. Mol. Life Sci. 64, 632–640 (2007). https://doi.org/10.1007/s00018-007-6501-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-007-6501-8