Abstract
Objective and design
To determine whether repetitive airway Pseudomonas aeruginosa (Pa) infection results in lung inflammation and injury and, if so, whether these responses are affected by Muc1 mucin. Muc1 wild type (WT) and knockout (KO) mice were compared for body weights, lung inflammatory responses, and airspace enlargement using a chronic lung infection model system.
Materials
Mice were treated intranasally with Pa (107 CFU) on days 0, 4, 7 and 10. On day 14, body weights, inflammatory cell numbers in bronchoalveolar lavage fluid (BALF), and airspace enlargement were measured. Differences in inflammatory responses between groups were statistically analyzed by the Student’s t test and ANOVA.
Results
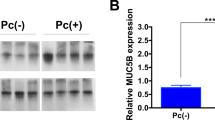
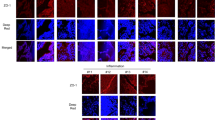
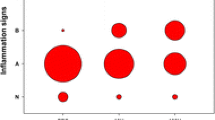
Muc1 WT mice exhibited mild degrees of both inflammation and airspace enlargement following repetitive airway Pa infection. However, Muc1 KO mice exhibited significantly decreased body weights, greater macrophage numbers in the BALF, and increased airspace enlargement compared with Muc1 WT mice.
Conclusions
This is the first report demonstrating that Muc1 deficiency can lead to lung injury during chronic Pa infection in mice. These results suggest that MUC1 may play a crucial role in the resolution of inflammation during chronic respiratory infections and that MUC1 dysfunction likely contributes to the pathogenesis of chronic inflammatory respiratory disease.





Similar content being viewed by others
References
Levison ME, Kaye D. Pneumonia caused by gram-negative bacilli: an overview. Rev Infect Dis 1985;7 Suppl 4:S656–65.
Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168(8):918–51.
Afessa B, Green B. Bacterial pneumonia in hospitalized patients with HIV infection: the Pulmonary Complications, ICU Support, and Prognostic Factors of Hospitalized Patients with HIV (PIP) Study. Chest. 2000;117(4):1017–22.
Eller J, Ede A, Schaberg T, Niederman MS, Mauch H, Lode H. Infective exacerbations of chronic bronchitis: relation between bacteriologic etiology and lung function. Chest. 1998;113(6):1542–8.
Soler N, Ewig S, Torres A, Filella X, Gonzalez J, Zaubet A. Airway inflammation and bronchial microbial patterns in patients with stable chronic obstructive pulmonary disease. Eur Respir J. 1999;14(5):1015–22.
Miravitlles M, Espinosa C, Fernandez-Laso E, Martos JA, Maldonado JA, Gallego M. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Study Group of Bacterial Infection in COPD. Chest. 1999;116(1):40–6.
Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347(7):465–71.
Groenewegen KH, Wouters EF. Bacterial infections in patients requiring admission for an acute exacerbation of COPD; a 1-year prospective study. Respir Med. 2003;97(7):770–7.
Eidelman D, Saetta MP, Ghezzo H, Wang NS, Hoidal JR, King M, et al. Cellularity of the alveolar walls in smokers and its relation to alveolar destruction: functional implications. Am Rev Respir Dis. 1990;141(6):1547–52.
Finkelstein R, Fraser RS, Ghezzo H, Cosio MG. Alveolar inflammation and its relation to emphysema in smokers. Am J Respir Crit Care Med. 1995;152(5 Pt 1):1666–72.
Di Stefano A, Capelli A, Lusuardi M, Balbo P, Vecchio C, Maestrelli P, et al. Severity of airflow limitation is associated with severity of airway inflammation in smokers. Am J Respir Crit Care Med. 1998;158(4):1277–85.
Betsuyaku T, Nishimura M, Takeyabu K, Tanino M, Venge P, Xu S, et al. Neutrophil granule proteins in bronchoalveolar lavage fluid from subjects with subclinical emphysema. Am J Respir Crit Care Med. 1999;159(6):1985–91.
Tetley TD. Macrophages and the pathogenesis of COPD. Chest. 2002;121(5 Suppl):156S–9S.
Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277(5334):2002–4.
Owen CA. Roles for proteinases in the pathogenesis of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2008;3(2):253–68.
Gendler SJ. MUC1, the renaissance molecule. J Mammary Gland Biol Neoplasia. 2001;6(3):339–53.
Hirasawa Y, Kohno N, Yokoyama A, Inoue Y, Abe M, Hiwada K. KL-6, a human MUC1 mucin, is chemotactic for human fibroblasts. Am J Respir Cell Mol Biol. 1997;17(4):501–7.
Lu W, Hisatsune A, Koga T, Kato K, Kuwahara I, Lillehoj EP, et al. Cutting edge: enhanced pulmonary clearance of Pseudomonas aeruginosa by Muc1 knockout mice. J Immunol. 2006;176(7):3890–4.
Lillehoj EP, Hyun SW, Kim BT, Zhang XG, Lee DI, Rowland S, et al. Muc1 mucins on the cell surface are adhesion sites for Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol. 2001;280(1):L181–7.
Kato K, Lillehoj EP, Kai H, Kim KC. MUC1 expression by human airway epithelial cells mediates Pseudomonas aeruginosa adhesion. Front Biosci (Elite Ed). 2010;1(2):68–77.
Lillehoj EP, Kim H, Chun EY, Kim KC. Pseudomonas aeruginosa stimulates phosphorylation of the airway epithelial membrane glycoprotein Muc1 and activates MAP kinase. Am J Physiol Lung Cell Mol Physiol. 2004;287(4):L809–15.
Choi S, Park YS, Koga T, Treloar A, Kim KC. TNF-alpha is a key regulator of MUC1, an anti-inflammatory molecule, during airway Pseudomonas aeruginosa infection. Am J Respir Cell Mol Biol. 2011;44(2):255–60.
Spicer AP, Rowse GJ, Lidner TK, Gendler SJ. Delayed mammary tumor progression in Muc-1 null mice. J Biol Chem. 1995;270(50):30093–101.
Ho MK, Springer TA. Tissue distribution, structural characterization, and biosynthesis of Mac-3, a macrophage surface glycoprotein exhibiting molecular weight heterogeneity. J Biol Chem. 1983;258(1):636–42.
Flotte TJ, Springer TA, Thorbecke GJ. Dendritic cell and macrophage staining by monoclonal antibodies in tissue sections and epidermal sheets. Am J Pathol. 1983;111(1):112–24.
Kato K, Lillehoj EP, Park YS, Umehara T, Hoffman NE, Madesh M, et al. Membrane-Tethered MUC1 Mucin Is Phosphorylated by Epidermal Growth Factor Receptor in Airway Epithelial Cells and Associates with TLR5 To Inhibit Recruitment of MyD88. J Immunol. 2012;16(188):2014–22.
Dunnill MS. Quantitative methods in the study of pulmonary pathology. Thorax. 1962;17:320–8.
Li Y, Dinwiddie DL, Harrod KS, Jiang Y, Kim KC. Anti-inflammatory effect of MUC1 during respiratory syncytial virus infection of lung epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol. 2010;298(4):L558–63.
van Heeckeren AM, Tscheikuna J, Walenga RW, Konstan MW, Davis PB, Erokwu B, et al. Effect of Pseudomonas infection on weight loss, lung mechanics, and cytokines in mice. Am J Respir Crit Care Med. 2000;161(1):271–9.
Lagente V, Le Quement C, Boichot E. Macrophage metalloelastase (MMP-12) as a target for inflammatory respiratory diseases. Expert Opin Ther Targets. 2009;13(3):287–95.
Kim KC, Lillehoj EP. MUC1 mucin: a peacemaker in the lung. Am J Respir Cell Mol Biol. 2008;39(6):644–7.
Cash HA, Woods DE, McCullough B, Johanson WG Jr, Bass JA. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. Am Rev Respir Dis. 1979;119(3):453–9.
Starke JR, Edwards MS, Langston C, Baker CJ. A mouse model of chronic pulmonary infection with Pseudomonas aeruginosa and Pseudomonas cepacia. Pediatr Res. 1987;22(6):698–702.
Heeckeren A, Walenga R, Konstan MW, Bonfield T, Davis PB, Ferkol T. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa. J Clin Invest. 1997;100(11):2810–5.
Koga T, Kuwahara I, Lillehoj EP, Lu W, Miyata T, Isohama Y, et al. TNF-alpha induces MUC1 gene transcription in lung epithelial cells: its signaling pathway and biological implication. Am J Physiol Lung Cell Mol Physiol. 2007;293(3):L693–701.
Zhang Z, Louboutin JP, Weiner DJ, Goldberg JB, Wilson JM. Human airway epithelial cells sense Pseudomonas aeruginosa infection via recognition of flagellin by Toll-like receptor 5. Infect Immun. 2005;73(11):7151–60.
Ueno K, Koga T, Kato K, Golenbock DT, Gendler SJ, Kai H, et al. MUC1 mucin is a negative regulator of toll-like receptor signaling. Am J Respir Cell Mol Biol. 2008;38(3):263–8.
Kato K, Lu W, Kai H, Kim KC. Phosphoinositide 3-kinase is activated by MUC1 but not responsible for MUC1-induced suppression of Toll-like receptor 5 signaling. Am J Physiol Lung Cell Mol Physiol. 2007;293(3):L686–92.
Kyo Y, Kato K, Park YS, Gajhate S, Umehara T, Lillehoj EP, et al. Anti-inflammatory role of MUC1 mucin during nontypeable Haemophilus influenzae infection. Am J Respir Cell Mol Biol. 2012;46(2):149–56.
Jarrard JA, Linnoila RI, Lee H, Steinberg SM, Witschi H, Szabo E. MUC1 is a novel marker for the type II pneumocyte lineage during lung carcinogenesis. Cancer Res. 1998;58(23):5582–9.
Nagai A, Inano H, Matsuba K, Thurlbeck WM. Scanning electronmicroscopic morphometry of emphysema in humans. Am J Respir Crit Care Med. 1994;150(5 Pt 1):1411–5.
Snider GL. Emphysema: the first two centuries–and beyond: A historical overview, with suggestions for future research: Part 1. Am Rev Respir Dis. 1992;146(5 Pt 1):1334–44.
Barnes PJ. Medicine. Neutrophils find smoke attractive. Science. 2010;330(6000):40–1.
Russell REK, Thorley A, Culpitt SV, Dodd S, Donnelly LE, Demattos C, et al. Alveolar macrophage-mediated elastolysis: roles of matrix metalloproteinases, cysteine, and serine proteases. Am J Physiol—Lung Cell Mol Physiol. 2002;283(4):L867–73.
Shapiro SD, Goldstein NM, Houghton AM, Kobayashi DK, Kelley D, Belaaouaj A. Neutrophil elastase contributes to cigarette smoke-induced emphysema in mice. Am J Pathol. 2003;163(6):2329–35.
Leco KJ, Waterhouse P, Sanchez OH, Gowing KL, Poole AR, Wakeham A, et al. Spontaneous air space enlargement in the lungs of mice lacking tissue inhibitor of metalloproteinases-3 (TIMP-3). J Clin Invest. 2001;108(6):817–29.
da Hora K, Valenca SS, Porto LC. Immunohistochemical study of tumor necrosis factor-alpha, matrix metalloproteinase-12, and tissue inhibitor of metalloproteinase-2 on alveolar macrophages of BALB/c mice exposed to short-term cigarette smoke. Exp Lung Res. 2005;31(8):759–70.
Pons AR, Sauleda J, Noguera A, Pons J, Barcelo B, Fuster A, et al. Decreased macrophage release of TGF-beta and TIMP-1 in chronic obstructive pulmonary disease. Eur Respir J. 2005;26(1):60–6.
Taraseviciene-Stewart L, Douglas IS, Nana-Sinkam PS, Lee JD, Tuder RM, Nicolls MR, et al. Is alveolar destruction and emphysema in chronic obstructive pulmonary disease an immune disease? Proc Am Thorac Soc. 2006;3(8):687–90.
Aoshiba K, Yokohori N, Nagai A. Alveolar wall apoptosis causes lung destruction and emphysematous changes. Am J Respir Cell Mol Biol. 2003;28(5):555–62.
Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, et al. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000;106(11):1311–9.
Mahoney JA, Rosen A. Apoptosis and autoimmunity. Curr Opin Immunol. 2005;17(6):583–8.
Agata N, Ahmad R, Kawano T, Raina D, Kharbanda S, Kufe D. MUC1 oncoprotein blocks death receptor-mediated apoptosis by inhibiting recruitment of caspase-8. Cancer Res. 2008;68(15):6136–44.
Kuwahara I, Lillehoj EP, Koga T, Isohama Y, Miyata T, Kim KC. The signaling pathway involved in neutrophil elastase stimulated MUC1 transcription. Am J Respir Cell Mol Biol. 2007;37(6):691–8.
Guo X, Verkler TL, Chen Y, Richter PA, Polzin GM, Moore MM, et al. Mutagenicity of 11 cigarette smoke condensates in two versions of the mouse lymphoma assay. Mutagenesis. 2011;26(2):273–81.
Pleasance ED, Stephens PJ, O’Meara S, McBride DJ, Meynert A, Jones D, et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature. 2010;463(7278):184–90.
Acknowledgments
This work was supported by grants from National Institute of Health, HL-47125 and HL-81825 (KCK).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Michael Parnham.
T. Umehara and K. Kato contributed equally to this work.
Rights and permissions
About this article
Cite this article
Umehara, T., Kato, K., Park, Y.S. et al. Prevention of lung injury by Muc1 mucin in a mouse model of repetitive Pseudomonas aeruginosa infection. Inflamm. Res. 61, 1013–1020 (2012). https://doi.org/10.1007/s00011-012-0494-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-012-0494-y