Abstract
Objective and design
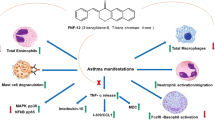
Although treatment for asthma control has improved a lot recently, refractory asthma is still a challenge for clinicians. Evidence revealed that anti-tumor necrosis factor (TNF)-α therapy may have potential in treating refractory asthma. Recently in an animal model, prostaglandin I2 (PGI2) analogues can suppress the cardinal feature of asthma. However, whether PGI2 analogues can regulate TNF-α expression in monocytes and the mechanism is not well-known.
Materials and methods
The human monocytes were pretreated with beraprost, iloprost and treprostinil, three PGI2 analogues, before stimulation with lipopolysaccharide (LPS). TNF-α concentration of the cell supernatants was measured by ELISA. Intracellular signaling was investigated by Western blot.
Results
PGI2 analogues suppressed LPS-induced TNF-α expression in THP-1 cells. CAY10449, an I prostanoid receptor antagonist, could reverse these effects. Beraprost increased intracellular cAMP level in THP1 cells. Forskolin, an adenylyl cyclase activator, could confer similar effect. LPS-induced TNF-α expression in THP-1 cells could be reversed by mitogen-activator protein kinase (MAPK)-p38, extracellular signal-related kinase (ERK) and c-Jun N-terminal kinase (JNK) inhibitors. Western blot revealed that beraprost suppressed MAPK phospho-p38, phosphor-JNK and phosphor-ERK expression.
Conclusion
PGI2 analogues suppressed LPS-induced TNF-α expression in THP-1 cells via the IP receptor-cAMP and the MAPK pathways. PGI2 analogues may have potentiality to treat asthma.





Similar content being viewed by others
References
Howarth PH, Babu KS, Arshad HS, Lau L, Buckley M, McConnell W, et al. Tumour necrosis factor (TNFalpha) as a novel therapeutic target in symptomatic corticosteroid dependent asthma. Thorax. 2005;60:1012–8.
Broide DH, Lotz M, Cuomo AJ, Coburn DA, Federman EC, Wasserman SI. Cytokines in symptomatic asthma airways. J Allergy Clin Immunol. 1992;89:958–67.
Brightling C, Berry M, Amrani Y. Targeting TNF-alpha: a novel therapeutic approach for asthma. J Allergy Clin Immunol. 2008;121:5–10. quiz 1–2.
Franchimont D, Martens H, Hagelstein MT, Louis E, Dewe W, Chrousos GP, et al. Tumor necrosis factor alpha decreases, and interleukin-10 increases, the sensitivity of human monocytes to dexamethasone: potential regulation of the glucocorticoid receptor. J Clin Endocrinol Metab. 1999;84:2834–9.
Berry MA, Hargadon B, Shelley M, Parker D, Shaw DE, Green RH, et al. Evidence of a role of tumor necrosis factor alpha in refractory asthma. N Engl J Med. 2006;354:697–708.
Badesch DB, McLaughlin VV, Delcroix M, Vizza CD, Olschewski H, Sitbon O, et al. Prostanoid therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:56S–61S.
Nagao K, Tanaka H, Komai M, Masuda T, Narumiya S, Nagai H. Role of prostaglandin I2 in airway remodeling induced by repeated allergen challenge in mice. Am J Respir Cell Mol Biol. 2003;29:314–20.
Idzko M, Hammad H, van Nimwegen M, Kool M, Vos N, Hoogsteden HC, et al. Inhaled iloprost suppresses the cardinal features of asthma via inhibition of airway dendritic cell function. J Clin Invest. 2007;117:464–72.
Hung CH, Chu YT, Suen JL, Lee MS, Chang HW, Lo YC, et al. Regulation of cytokine expression in human plasmacytoid dendritic cells by prostaglandin I2 analogues. Eur Respir J. 2009;33:405–10.
Czeslick EG, Simm A, Grond S, Silber RE, Sablotzki A. Inhibition of intracellular tumour necrosis factor (TNF)-alpha and interleukin (IL)-6 production in human monocytes by iloprost. Eur J Clin Invest. 2003;33:1013–7.
Kuo CH, Ko YC, Yang SN, Chu YT, Wang WL, Huang SK, et al. Effects of PGI(2) analogues on Th1- and Th2-related chemokines in monocytes via epigenetic regulation. J Mol Med. 2011;89:29–41.
Straus DS, Glass CK. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 2007;28:551–8.
Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94.
Hung CH, Suen JL, Hua YM, Chiang W, Chang HC, Chen CN, et al. Suppressive effects of ketotifen on Th1- and Th2-related chemokines of monocytes. Pediatr Allergy Immunol. 2007;18:378–84.
Serra-Batlles J, Plaza V, Morejon E, Comella A, Brugues J. Costs of asthma according to the degree of severity. Eur Respir J. 1998;12:1322–6.
Burgess JK, Ge Q, Boustany S, Black JL, Johnson PR. Increased sensitivity of asthmatic airway smooth muscle cells to prostaglandin E2 might be mediated by increased numbers of E-prostanoid receptors. J Allergy Clin Immunol. 2004;113:876–81.
Jaffar Z, Ferrini ME, Buford MC, Fitzgerald GA, Roberts K. Prostaglandin I2-IP signaling blocks allergic pulmonary inflammation by preventing recruitment of CD4+ Th2 cells into the airways in a mouse model of asthma. J Immunol. 2007;179:6193–203.
Aronoff DM, Peres CM, Serezani CH, Ballinger MN, Carstens JK, Coleman N, et al. Synthetic prostacyclin analogs differentially regulate macrophage function via distinct analog-receptor binding specificities. J Immunol. 2007;178:1628–34.
Gomberg-Maitland M, Olschewski H. Prostacyclin therapies for the treatment of pulmonary arterial hypertension. Eur Respir J. 2008;31:891–901.
Zhou W, Hashimoto K, Goleniewska K, O’Neal JF, Ji S, Blackwell TS, et al. Prostaglandin I2 analogs inhibit proinflammatory cytokine production and T cell stimulatory function of dendritic cells. J Immunol. 2007;178:702–10.
Acknowledgments
This study was supported by a grant from Kaohsiung Medical University Hospital KMUH-98-8G09 and KMUH-99-9I08, and a grant from Kaohsiung Municipal Ta-Tung Hospital KMTTH-99-012. The project is supported by a grant from the Center of Excellence for Environmental Medicine Kaohsiung Medical University Research Foundation KMU-EM-98-4.1.
Conflict of interest
The authors declared that no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Liwu Li.
Rights and permissions
About this article
Cite this article
Wang, WL., Kuo, CH., Chu, YT. et al. Prostaglandin I2 analogues suppress TNF-α expression in human monocytes via mitogen-activated protein kinase pathway. Inflamm. Res. 60, 655–663 (2011). https://doi.org/10.1007/s00011-011-0317-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-011-0317-6