Abstract
Objective
It is an open question whether multifunctional galectin-3 can be a serum marker in inflammatory bowel disease.
Methods
Western blots and commercial ELISA detected and quantitated the lectin immunocytochemistry using double labeling localized it in tissue sections.
Results
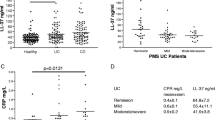
Serum concentrations were significantly increased in specimen of patients with active and remission-stage ulcerative colitis and Crohn’s disease, associated with emerging positivity of CD14+ cells.
Conclusion
Enhanced concentration of galectin-3 in serum reflects presence of disease and points to its involvement in the pathogenesis.










Similar content being viewed by others
References
Gabius H-J. Cell surface glycans: the why and how of their functionality as biochemical signals in lectin-mediated information transfer. Crit Rev Immunol. 2006;26:43–79.
Villalobo A, Nogales-González A, Gabius H-J. A guide to signaling pathways connecting protein-glycan interaction with the emerging versatile effector functionality of mammalian lectins. Trends Glycosci Glycotechnol. 2006;18:1–37.
Dimic J, Dabelic S, Flögel M. Galectin-3: and open-ended story. Biochim Biophys Acta. 2006;1760:616–35.
Gabius HJ. Animal lectins. Eur J Biochem. 1997;243:543–76.
Ahmad N, Gabius H-J, André S, Kaltner H, Sabesan S, Roy R, et al. Galectin-3 precipitates as a pentamer with synthetic carbohydrates and forms heterogeneous cross-linked complexes. J Biol Chem. 2004;279:10841–7.
Almkvist J, Karlsson A. Galectins as inflammatory mediators. Glycoconj J. 2004;19:575–81.
Kübler D, Hung C-W, Dam TK, Kopitz J, André S, Kaltner H, et al. Phosphorylated human galectin-3: facile large-scale preparation of active lectin and detection of structural changes by CD spectroscopy. Biochim Biophys Acta. 2008;1780:716–22.
Shanahan F. Inflammatory bowel disease: immunodiagnostics, immunotherapeutics, and ecotherapeutics. Gastroenterology. 2001;120:622–35.
Hibi T, Ogata H. Novel pathophysiological concepts of inflammatory bowel disease. J Gastroenterol. 2006;41:10–6.
Scaldaferri F, Fiocchi C. Inflammatory bowel disease: progress and current concepts of etiopathogenesis. J Dig Dis. 2007;8:171–8.
Iurisci I, Tinari N, Natoli C, Angelucci D, Cianchetti E, Iacobelli S. Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin Cancer Res. 2000;6:1389–93.
Jensen-Jarolin E, Neumann C, Oberhuber G, Gscheidlinger R, Neuchrist C, Reinisch W, et al. Anti-galectin-3 IgG autoantobodies in patients with Crohn’s disease characterized by means of phage display peptide libraries. J Clin Immunol. 2001;21:348–56.
Best WR. Predicting the Crohn’s disease activity index from the Harvey-Bradshaw Index. Inflamm Bowel Dis. 2006;12:304–10.
Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–76.
Schroeder KW, Tremaine WJ, Ilstrup DM (1987) Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 24;317:1625-9.
Kopitz J, von Reitzenstein C, André S, Kaltner H, Uhl J, Ehemann V, et al. Negative regulation of neuroblastoma cell growth by carbohydrate- dependent surface binding of galectin-1 and functional divergence from galectin-3. J Biol Chem. 2001;276:35917–23.
Beer A, André S, Kaltner H, Lensch M, Franz S, Sarter K, et al. Human galectins as sensors for apoptosis/necrosis-associated surface changes of granulocytes and lymphocytes. Cytom Part A. 2008;73A:139–47.
Hudcovic T, Stepankova R, Cebra J, Tlaskalova-Hogenova H. The role of microflora in the development of intestinal inflammation: acute and chronic colitis induced by dextran sulfate in germ-free and conventionally reared immunocompetent and immunodeficient mice. Folia Microbiol (Praha). 2001;46:565–72.
Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–49.
Lodinova-Zadnikova R, Cukrowska B, Tlaskalova-Hogenova H. Oral administration of probiotic Escherichia coli after birth reduces frequency of allergies and repeated infections later in life (after 10 and 20 years). Int Arch Allergy Immunol. 2003;131:209–11.
Kwapinski JBG. Methodology of Immunochemical and Immunological Research. New York: Wiley; 1972. p. 605–7.
André S, Kojima S, Yamazaki N, Fink C, Kaltner H, Kayser K, et al. Galectins-1 and -3 and their ligands in tumor biology. J Cancer Res Clin Oncol. 1999;125:461–74.
Froňková V, Holíková Z, Liu F-T, Homolka J, Rijken DC, André S, et al. Simultaneous detection of endogenous lectins and their binding capability at the single-cell level: a technical note. Folia Biol (Praha). 1999;45:157–62.
Dam TK, Gabius H-J, André S, Kaltner H, Lensch M, Brewer CF. Galectins bind to the multivalent glycoprotein asialofetuin with enhanced affinities and a gradient of decreasing binding constants. Biochemistry. 2005;44:12564–71.
Gabius HJ, Schröter C, Gabius S, Brinck U, Tietze LF. Binding of T-antigen-bearing neoglycoprotein and peanut agglutinin to cultured tumor cells and breast carcinomas. J Histchem Cytochem. 1990;38:1625–31.
Gabius HJ, Gabius S, Zemlyanukhina TV, Bovin NV, Brinck U, Danguy A, et al. Reverse lectin histochemistry: design and application of glycoligands for detection of cell and tissue lectins. Histol Histopathol. 1993;8:369–83.
Plzák J, Betka J, Smetana K Jr, Chovanec M, Kaltner H, André S, et al. Galectin-3: an emerging prognostic indicator in advanced head and neck carcinoma. Eur J Cancer. 2004;40:2324–30.
Gabius HJ, Wosgien B, Hendrys M, Bardosi A. Lectin localization in human nerve by biochemically defined lectin-binding glycoproteins, neogycoprotein and lectin-specific antibody. Histochemistry. 1991;95:269–77.
Lohr M, Lensch M, André S, Kaltner H, Siebert HC, Smetana K Jr, et al. Murine homodimeric adhesion/growth-regulatory galectins-1, -2, and -7: comparative profiling of gene/promoter sequences by database mining, of expression by RT-PCR/immunohistochemsitry and of contact sites for carbohydrate ligands by computational chemistry. Folia Biol (Praha). 2007;53:109–28.
Jensen-Jarolim E, Gscheidlinger R, Oberhuber G, Neuchrist C, Lucas T, Bises G, et al. The constitutive expression of galectin-3 is downregulated in the intestinal epithelia of Crohn’s disease patients, and tumour necrosis factor-α decreases the level of galectin-3-specific mRNA in HCT-8 cells. Eur J Gastroenterol Hepatol. 2002;14:145–52.
Müller S, Schaffer T, Flogerzi B, Fleetwood A, Weimann R, Schoepfer AM, et al. Galectin-3 modulates T cell activity and is reduced in the inflamed intestinal epithelium in IBD. Inflamm Bowel Dis. 2006;12:588–97.
Shiobara N, Suzuki Y, Aoki H, Gotoh A, Fujii Y, Hamada Y, et al. Bacterial superantigens and T cell receptor β-chain-bearing T cells in the immunopathogenesis of ulcerative colitis. Clin Exp Immunol. 2007;150:13–21.
Saussez S, Glinoer D, Chantrain G, Pattou F, Carnaille B, André S, et al. Serum galectin-1 and galectin-3 levels in benign and malignant nodular thyroid disease. Thyroid. 2008;18:705–12.
Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702.
Hamdani G, Gabet Y, Rachmilewitz D, Karmeli F, Bab I, Dresner-Pollak R. Dextran sodium sulfate-induced colitis causes rapid bone loss in mice. Bone. 2008;43:945–50.
Subramanian S, Roberts CL, Hart CA, Martin HM, Edwards SW, Rhodes JM, et al. Replication of colonic crohn’s disease mucosal Escherichia coli isolates within macrophages and their susceptibility to antibiotics. Antimicrob Agents Chemother. 2008;52:427–34.
Tlaskalova-Hogenova H, Stepankova R, Hudcovic T, Tuckova L, Cukrowska B, Lodinova-Zadnikova R, et al. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol Lett. 2004;93:97–108.
Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–76.
Swidsinski A, Weber J, Loening-Baucke V, Hale LP, Lochs H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol. 2005;43:3380–9.
Sydora BC, Martin SM, Lupicki M, Dieleman LA, Doyle J, Walker JW, et al. Bacterial antigens alone can influence intestinal barrier integrity, but live bacteria are required for initiation of intestinal inflammation and injury. Inflamm Bowel Dis. 2006;12:429–36.
Stepankova R, Powrie F, Kofronova O, Kozakova H, Hudcovic T, Hrncir T, et al. Segmented filamentous bacteria in a defined bacterial cocktail induce intestinal inflammation in SCID mice reconstituted with CD45RBhigh CD4 + T cells. Inflamm Bowel Dis. 2007;13:1202–11.
Ogra PL, Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR. Mucosal Immunology. New York: Academic; 1999.
Walker WAJ. Development of the intestinal mucosal barrier. Pediatr Gastroenterol Nutr. 2002;34:S33–9.
Tlaskalová-Hogenová H, Tucková L, Stepánková R, Hudcovic T, Palová-Jelínková L, Kozáková H, et al. Involvement of innate immunity in the development of inflammatory and autoimmune diseases. Ann NY Acad Sci. 2005;1051:787–98.
Smith PD, Smythies LE, Mosteller-Barnum M, Sibley DA, Russell MW, Merger M, et al. Intestinal macrophages lack CD14 and CD89 and consequently are down-regulated for LPS- and IgA-mediated activities. J Immunol. 2001;167:2651–6.
Smith PD, Ochsenbauer-Jambor C, Smythies LE. Intestinal macrophages: unique effector cells of the innate immune system. Immunol Rev. 2005;206:149–59.
Rogler G, Andus T, Aschenbrenner E, Vogl D, Falk W, Scholmerich J, et al. Alterations of the phenotype of colonic macrophages in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1997;9:893–9.
MacDermott RP. Treatment of irritable bowel syndrome in outpatients with inflammatory bowel disease using a food and beverage intolerance, food and beverage avoidance diet. Inflamm Bowel Dis. 2007;13:91–6.
MacDonald TT, Gordon JN. Bacterial regulation of intestinal immune responses. Gastroenterol Clin North Am. 2005;34:401–12.
Lohr M, Kaltner H, Lensch M, Andre S, Sinowatz F, Gabius HJ. Cell-type-specific expression of murine multifunctional galectin-3 and its association with follicular atresia/luteolysis in contrast to pro-apoptic galectins-1 and -7. Histochem Cell Biol. 2008;130:567–81.
Sano H, Hsu DK, Yu L, Apgar JR, Kuwabara I, Yamanaka T, et al. Human galectin-3 is a novel chemoattractant for monocytes and macrophages. J Immunol. 2000;165:2156–64.
André S, Sanchez-Ruderisch H, Nakagawa H, Buchholz M, Koptiz J, Forberich P, et al. Tumor suppressor p16INK4a–modulator of glycomic profile and galectin-1 expression to increase susceptibility to carbohydrate-dependent induction of anoikis in pancreatic carcinoma cells. FEBS J. 2007;272:3233–56.
Schwartz-Albiez R. Inflammation and glycosciences. In: Gabius HJ, editor. The sugar code. Fundamentals of glycosciences. Weinheim: Wiley; 2009 (in press).
Sharma U, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JPM, Schroen B, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121–8.
Acknowledgments
This work was supported by the Czech Science Foundation (project nos. 310/08/H077 and 303/060/974), the Academy of Sciences (project no. S500200572), the Ministry of Education, Youth and Sports of the Czech Republic (projects no. 2B06155, no. MSM0021620806 and 1M0538), the Institute of Microbiology (project no. AV0Z50200510), the Ministry of Health (project no. NR8963-3), the research initiative LMUexcellent, the Verein zur Förderung des biologisch-technologischen Fortschritts in der Medizin e. V. and an EU Marie Curie Research Training Network grant (contract no. MCRTN-CT-2005-19561). The authors are grateful to B. Hofmanová and I. Burdová for excellent technical assistance, Dr. B. Friday and Dr. S. Namirha for valuable discussion and to the reviewers of this manuscript for their inspiring advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: I. Ahnfelt-Rønne.
Rights and permissions
About this article
Cite this article
Frol’ová, L., Smetana, K., Borovská, D. et al. Detection of galectin-3 in patients with inflammatory bowel diseases: new serum marker of active forms of IBD?. Inflamm. Res. 58, 503–512 (2009). https://doi.org/10.1007/s00011-009-0016-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-009-0016-8