Abstract
Background
The efficacy and safety of Symbicort®1 (budesonide and formoterol in a single inhaler) were compared with those of a high dose of the commonly used corticosteroid fluticasone propionate in patients with moderate persistent asthma.
Methods
This randomized, double-blind, double-dummy, parallel-group study involved 373 patients with asthma (mean age 42 years; FEV1 78% of predicted; reversibility 21%). After a 2-week run-in period, during which patients received budesonide 200μg twice daily, they were randomly assigned to treatment with either Symbicort® Turbuhaler® (budesonide/formoterol 160/4.5μg, one inhalation twice daily) or Flovent®/Flixotide® Diskus™ (fluticasone propionate 250μg twice daily) for 12 weeks.
Results
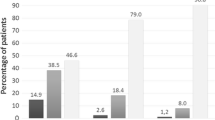
Significantly greater increases in morning PEF, the primary efficacy variable, were observed in patients treated with budesonide/formoterol compared with fluticasone propionate (27.4 L/min vs 7.7 L/min; p < 0.001). Evening PEF and clinic FEV1 also favored budesonide/formoterol compared with fluticasone propionate (p < 0.001), as did use of reliever medication (p = 0.04) and the proportion of reliever-free days (p < 0.001). There were also numerical improvements in symptom-free days (60.4% vs 55.5%), night-time awakenings (7.9% vs 9.6%) and asthma-control days (57.8% vs 52.4%) in favor of budesonide/formoterol. The risk of an exacerbation was reduced by 32% in the budesonide/formoterol group compared with the fluticasone propionate group (p < 0.05). Both treatments were well tolerated.
Conclusion
Symbicort® (budesonide/formoterol in a single inhaler) was more effective than a high dose of fluticasone propionate in improving lung function, reducing use of reliever medication and improving control of moderate persistent asthma.






Similar content being viewed by others
References
National Institutes of Health: National Heart Lung and Blood Institute. Global Initiative for Asthma. Global strategy for asthma management and prevention. NHLBI/WHO Workshop Report. Report no. NIH-NHLI 95-3659. Bethesda (MD): National Institutes of Health National Heart and Lung Institute; 1995
National Institutes of Health: National Heart Lung and Blood Institute. National Asthma Education and Prevention Program. Expert panel report II: guidelines for the diagnosis and management of asthma. Report no. NIH-NHLI 97-4051. Bethesda (MD): National Institutes of Health National Heart and Lung Institute; 1997
National Institutes of Health: National Heart Lung and Blood Institute. Global Initiative for Asthma. Global strategy for asthma management and prevention. Report no. NIH-NHLI 02-3659. Bethesda (MD): National Institutes of Health National Heart and Lung Institute; 2002
Greening AP, Ind PW, Northfield M, et al. Added salmeterol versus higher-dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid. Lancet 1994; 344: 219–24
Woolcock A, Lundback B, Ringdal N, et al. Comparison of addition of salmeterol to inhaled steroids with doubling of the dose of inhaled steroids. Am J Respir Crit Care Med 1996; 153: 1481–8
Pauwels RA, Löfdahl C-G, Postma DS, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. N Engl J Med 1997; 337: 1405–11
Kips JC, O’Connor BJ, Inman MD, et al. A long-term study of the antiinflammatory effect of low-dose budesonide plus formoterol versus high-dose budesonide in asthma. Am J Respir Crit Care Med 2000; 161: 996–1001
Zetterström O, Buhl R, Mellem H, et al. Improved asthma control with budesonide/formoterol in a single inhaler, compared with budesonide alone. Eur Respir J 2001; 18: 262–8
Lalloo UG, Malolepszy J, Kozma D, et al. Symbicort® (budesonide and formoterol in a single inhaler) is more effective than increasing the dose of inhaled corticosteroids in mild asthma [abstract]. Am J Respir Crit Care Med 2001; 163: A863
Pauwels RA, Löfdahl C-G, Postma DS, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. N Engl J Med 1997; 337: 1405–11
O’Byrne PM, Barnes PJ, Rodriguez-Roison R, et al. Low dose inhaled budesonide with and without formoterol in steroid free patients with mild persistent asthma [abstract]. Am J Respir Crit Care Med 2001; 163: A862
Coutts JAP, Gibson NA, Paton JY. Measuring compliance with inhaled medication in asthma. Arch Dis Child 1992; 67: 332–3
Eisen SA, Miller DK, Woodward RS, et al. The effect of prescribed daily dose frequency on patient medication compliance. Arch Intern Med 1990; 150: 1881–4
Aziz l, Wilson AM, Lipworth BJ. Effects of once-daily formoterol and budesonide given alone or in combination on surrogate inflammatory markers in asthmatic adults. Chest 2000; 118: 1049–58
Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows: report of the working party standardization of lung function tests, European Community for Steel and Coal: Official Statement of the European Respiratory Society. Eur Respir J 1993; 6 Suppl. 16: 5–40
National Institutes of Health: National Heart Lung and Blood Institute. Global initiative for asthma: Pocket Guide for asthma management and prevention. Report no. NIH-NHLI 96-3659B. Bethesda (MD): National Institutes of Health National Heart and Lung Institute; 1998
Kavuru M, Melamed J, Gross G, et al. Salmeterol and fluticasone propionate combined in a new powder inhalation device for the treatment of asthma: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol 2000; 105(6 Pt 1): 1108–16
Shapiro G, Lumry W, Wolfe J, et al. Combined salmeterol 50μg and fluticasone propionate 250μg in the Diskus device for the treatment of asthma. Am J Respir Crit Care Med 2000; 161: 527–34
Jenkins C, Woolcock AJ, Saarelainen P, et al. Salmeterol/fluticasone propionate combination therapy 50/250 twice daily is more effective than budesonide 800μg twice daily in treating moderate to severe asthma. Respir Med 2000; 94: 715–23
Johansson G, Mclvor RA, D’Ambrosio FP, et al. Comparison of salmeterol/fluticasone propionate combination with budesonide in patients with mild-to-moderate asthma. Clin Drug Invest 2001; 21: 633–42
Dahl R, Lundback B, Malo J-L, et al. A dose-ranging study of fluticasone propionate in adult patients with moderate asthma. Chest 1993; 104: 1352–8
Busse WW, Chervinsky P, Condemi J, et al. Budesonide delivered by Turbuhaler® is effective in a dose-dependent fashion when used in the treatment of adult patients with chronic asthma. J Allergy Clin Immunol 1998; 101: 457–63
Acknowledgements
This study was sponsored by AstraZeneca, Lund, Sweden. Eric D. Bateman has received honoraria for consultancy activities and lectures from AstraZeneca, GSK, Boehringer Ingelheim, Kyowa Hakko and Pfizer. Rudolf M. Huber has received reimbursement for attending international conferences, and fees for speaking over the last 5 years from GSK, AstraZeneca and Altana. Ian Naya is a full-time employee of AstraZeneca. Theo A. Bantje, Maria Joao Gomes, Michael G. Toumbis and Avraham Eliraz have no competing interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bateman, E.D., Bantje, T.A., Gomes, M.J. et al. Combination Therapy with Single Inhaler Budesonide/Formoterol Compared with High Dose of Fluticasone Propionate Alone in Patients with Moderate Persistent Asthma. Am J Respir Med 2, 275–281 (2003). https://doi.org/10.1007/BF03256655
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03256655