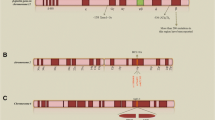
Abstract
As the defective genes for more and more genetic disorders become unravelled, it is clear that patients with the same genotype can have many different clinical conditions even in monogenic disorders. The remarkable phenotypic diversity of the βthalassaemias is prototypical of how the wide spectrum in disease severity can be generated. The most reliable and predictive factor of disease phenotype is the nature of the mutation at the β-globin locus itself. However, relating phenotype to genotype is complicated by the complex interaction of the environment and other genetic factors at the secondary and tertiary levels, some implicated, and others, as yet unidentified. This article reviews the clinical and haematological diversity encountered in βthalassaemia and their relationship with the under-lying genotypes.
Similar content being viewed by others
References
Weatherall DJ, Clegg JB, editors. The Thalassaemia Syndromes. 4th ed. Oxford: Blackwell Science; 2001.
Forget BG. Molecular Genetics of the Human Globin Genes. In: Steinberg MH, Forget BG, Higgs DR, Nagel RL, editors. Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management. Cambridge, UK: Cambridge University Press; 2001. p. 117–130.
Stamatoyannopoulos G. Molecular and Cellular Basis of Hemeoglobin Switching. In: Steinberg MH, Forget BG, Higgs DR, Nagel RL, editors. Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management. Cambridge, UK: Cambridge University Press; 2001. p. 131–145.
Schrier SL. Pathophysiology of thalassemia.Curr Opin Hematol. 2002;9:123–126.
Flint J, Harding RM, Boyce AJ, Clegg JB. The population genetics of the haemoglobinopathies. In: Rodgers GP, editor. Bailliére’s Clinical Haematology. London: Bailliere Tindall; 1998. p. 1–52.
Thein SL. Baillières Clinical Haematology: Beta thalassaemia in sickle cell disease and thalassaemia. In: Rodgers GP, editor. Sickle Cell Disease and Thalassaemia. London: Baillire Tindall; 1998. p. 91–126.
Viprakasit V, Gibbons RJ, Broughton BC, et al. Mutations in the general transcription factor TFIIH result in beta-thalassaemia in individuals with trichothiodystrophy.Hum Mol Genet. 2001;10:2797–2802.
Huisman THJ, Carver MFH, Efremov GD. A Syllabus of Human Hemoglobin Variants. 2nd ed. Augusta, GA, USA: The Sickle Cell Anemia Foundation; 1998.
Divoky V, Indrak K, Mrug M, Brabec V, Huisman THJ, Prchal JT. A novel mechanism of β thalassemia: the insertion of L1 retrotransposable element into β globin IVS II.Blood. 1996;88:148a.
Maquat LE. When cells stop making sense: effects of nonsense codons on RNA metabolism in vertebrate cells.RNA. 1995;1:453–465.
Maquat LE, Carmichael GG. Quality control of mRNA function.Cell. 2001;104:173–176.
Rund D, Filon D, Strauss N, Rachmilewitz EA, Oppenheim A. Mean corpuscular volume of heterozygotes for β-thalassemia correlates with the severity of mutations.Blood. 1991;79:238–243.
Maragoudaki E, Kanavakis E, Trager-Synodinos J, et al. Molecular, haematological and clinical studies of the −101 C->T substitution in the β-globin gene promoter in 25 β-thalassaemia intermedia patients and 45 heterozygotes.Br J Haematol. 1999;107:699–706.
Ho PJ, Hall GW, Luo LY, Weatherall DJ, Thein SL. Beta thalassemia intermedia: is it possible to consistently predict phenotype from genotype?Br J Haematol. 1998;100:70–78.
Camaschella C, Maza U, Roetto A, et al. Genetic interactions in thalassemia intermedia: analysis of β-mutations, α-genotype, γ-promoters, and β- LCR hypersensitive sites 2 and 4 in Italian patients.Am J Hematol. 1995;48:82–87.
Thein SL, Hesketh C, Wallace RB, Weatherall DJ. The molecular basis of thalassaemia major and thalassaemia intermedia in Asian Indians: application to prenatal diagnosis.Br J Haematol. 1988;70:225–231.
Craig JE, Kelly SJ, Barnetson R, Thein SL. Molecular characterization of a novel 10.3 kb deletion causing β-thalassaemia with unusually high Hb A2.Br J Haematol. 1992; 82:735–744.
Thein SL, Hesketh C, Taylor P, et al. Molecular basis for dominantly inherited inclusion body β-thalassemia. Proceedings of the National Academy of Sciences.USA. 1990;87:3924–3928.
Thein SL. Dominant β thalassaemia: molecular basis and pathophysiology.Br J Haematol. 1992;80:273–277.
Thein SL. Is it dominantly inherited b thalassaemia or just a β-chain variant that is highly unstable?Br J Haematol. 1999;107:12–21.
Ho PJ, Wickramasinghe SN, Rees DC, Lee MJ, Eden A, Thein SL. Erythroblastic inclusions in dominantly inherited β thalassaemias.Blood. 1997;89:322–328.
Hentze MW, Kulozik AE. A perfect message: RNA surveillance and nonsense-mediated decay.Cell. 1999;96:307–310.
Thein SL. Structural Variants with a b-Thalassemia Phenotype. In: Steinberg MH, Forget BG, Higgs DR, Nagel RL, editors. Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management. Cambridge, UK: Cambridge University Press, Cambridge, UK; 2001. p. 342–355.
Bunn HF, Forget BG. Hemoglobin: Molecular, Genetic and Clinical Aspects. Philadelphia, PA: W. B. Saunders Company, 1986.
Camaschella C, Kattamis AC, Petroni D, et al. Different hematological phenotypes caused by the interaction of triplicatted α-globin genes and heterozygous β-thalassemia.Am J Hematol. 1997;55:83–88.
Traeger-Synodinos J, Kanavakis E, Vrettou C, Maragoudaki E, Michael T, Metaxotou-Mavromati A. The triplicated α-globin gene locus in β-thalassaemia heterozygotes: clinical, haematological, biosynthetic and molecular studies.Br J Haematol. 1996;95:467–471.
Garner C, Tatu T, Reittie JE, et al. Genetic influences on F cells and other hematological variables: a twin heritability study.Blood. 2000;95:342–346.
Garner C, Tatu T, Game L, et al. A candidate gene study of F cell levels in sibling pairs using a joint linkage and association analysis.GeneScreen. 2000;1:9–14.
Wood WG. Hereditary Persistence of Fetal Hemoglobin and δβ Thalassemia. In: Steinberg MH, Forget BG, Higgs DR, Nagel RL, editors. Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management. Cambridge, UK: Cambridge University Press, Cambridge, UK; 2001. p. 356–388.
Galanello R, Dessi E, Melis MA, et al. Molecular analysis of βo-thalassemia intermedia in Sardinia.Blood. 1989;74:823–827.
Craig JE, Rochette J, Fisher CA, et al. Dissecting the loci controlling fetal haemoglobin production on chromosomes 11p and 6q by the regressive approach.Nature Genetics. 1996; 12:58–64.
Craig JE, Rochette J, Sampietro M, et al. Genetic heterogeneity in heterocellular hereditary persistence of fetal hemoglobin.Blood. 1997;90:428–434.
Dover GJ, Smith KD, Chang YC, et al. Fetal hemoglobin levels in sickle cell disease and normal individuals are partially controlled by an X-linked gene located at Xp22.2Blood. 1992;80:816–824.
Garner CP, Tatu T, Best S, Creary L, Thein SL. Evidence for Genetic Interaction between the beta-globin complex and chromosome 8q in the expression of fetal hemoglobin.Am J Hum Genet. 2002;70:793–799.
Thein SL, Craig JE. Genetics of Hb F/F cell variance in adults and heterocellular hereditary persistence of fetal hemoglobin.Hemoglobin. 1998;22:401–414.
Badens C, Mattei MG, Imbert AM, et al. A novel mechanism for thalassaemia intermedia.The Lancet. 2002;359:132–133.
Galanello R, Perseu L, Melis MA, et al. Hyperbilirubinaemia in heterozygous b- thalassaemia is related to co-inherited Gilbert’s syndrome.Br J Haematol. 1997;99:433–436.
Galanello R, Piras S, Barella S, et al. Cholelithiasis and Gilbert’s syndrome in homozygous β-thalassaemia.Br J Haematol. 2001;115:926–928.
Sampietro M, Lupica L, Perrero L, Comino A, Martinez di Montemuros F. The expression of uridine diphosphate glucuronosyltransferase gene is a major determinant of bilirubin level in heterozygous β-thalassaemia and in glucose-6-phosphate.Br J Haematol. 1997;99:437–439.
Bosma PJ, Chowdhury JR, Bakker C, et al. The genetic basis of the reduced expression of bilirubin UCP-glucuronosultransferase 1 in Gilbert’s syndrome.New England J Medicine. 1995;333:1171–1175.
Passon RG, Howard TA, Zimmerman SA, Schultz WH, Ware RE. Influence of Bilirubin Uridine Diphosphate- Glucuronosyltransferase 1A Promoter Polymorphisms on Serum Bilirubin Levels and Cholelithiasis in Children With Sickle Cell Anemia.Am J Pediatr Hematol Oncol. 2001;23:448–451.
Rees DC, Luo LY, Thein SL, Sing BM, Wickramasinghe S. Nontransfusional iron overload in thalassemia: Association with hereditary hemochromatosis.Blood. 1997;90:3234–3236.
Piperno A, Mariani R, Arosio C, et al. Haemochromatosis in patients with beta-thalassaemia trait.Br J Haematol. 2000;111:908–914.
Merryweather-Clarke AT, Pointon JJ, Shearman JD, Robson KJH. Global prevalence of putative haemochromatosis mutations.J Medical Genetics. 1997;34:275–278.
Andrews N. Iron homeostasis: insights from genetics and animal models.Nature Reviews Genetics. 2000;1:208–216.
Wonke B. Bone disease in b-thalassaemia major.Br J Haematol. 1998;103:897–901.
Dresner Pollack R, Rachmilewitz E, Blumenfeld A, Idelson M, Goldfarb AW. Bone mineral metabolism in adults with beta-thalassaemia major and intermedia.Br J Haematol. 2000; 111:902–907.
Economou-Peterson E, Aesspopos A, Kladi A, et al. Apolipoprotein E e4 allele as a genetic risk factor for left ventricular failure in homozygous β-thalassemia.Blood. 1998;92:3455–3459.
Author information
Authors and Affiliations
About this article
Cite this article
Thein, S.L. β-Thalassaemia prototype of a single gene disorder with multiple phenotypes. Int J Hematol 76 (Suppl 2), 96–104 (2002). https://doi.org/10.1007/BF03165097
Issue Date:
DOI: https://doi.org/10.1007/BF03165097