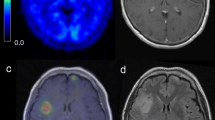
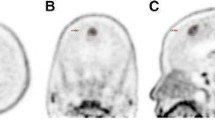
Abstract
Objective
The uptake of L-methyl-11C-methionine (MET) by gliomas is greater than that by intact tissue, making methionine very useful for evaluation of tumor extent. If the degree of malignancy of brain tumors can be evaluated by MET-PET, the usefulness of MET-PET as a means of diagnosing brain tumors will increase.
Methods
We performed this study on 67 glioma patients between 3 and 69 years of age (36 males and 31 females). Tumors included diffuse astrocytoma, anaplastic astrocytoma, glioblastoma, ependymoma, oligodendroglioma, medulloblastoma, dysembryoplastic neuroepithelial tumor, choroid plexus papilloma, central neurocytoma, optic glioma, gliomatosis cerebri, pleomorphic xanthoastrocytoma, and ganglioglioma. Tumor activity and degree of malignancy were evaluated using Ki-67LI (LI: labeling index) and Kaplan-Meier survival curves. The correlations between methionine uptake and tumor proliferation (tumor versus contralateral gray matter ratio (T/N) and Ki-67LI) were determined for the group of all subjects. The existence of significant correlations between T/N and KJ-67LI and between SUV and Ki-67LI was determined for astrocytic tumors. Receiver operating characteristics (ROC) analysis of T/N and standardized uptake value (SUV) was performed for the group of astrocytic tumors. We also determined the ROC cut-off levels to ensure high accuracy of the analysis.
Results
For the 67 cases of glioma, the degree of accumulation was variable. Ki-67LI differed significantly between the high-grade group and low-grade group at T/N levels between 1.5 and 1.8 on analysis using tumor proliferative potential (p - 0.019–0.031). The prognosis differed significantly between the highgrade and low-grade groups when T/N was in the range of 1.6–1.8 (p = 0.028–0.032). The accuracy thus calculated was highest (85.7%) when T/N was 1.5 as determined by ROC analysis.
Conclusions
When analysis was confined to cases of astrocytic tumor, a correlation was noted between methionine accumulation and Ki-67LI. For the astrocytic tumors, T/N ratio seemed to be more useful as a diagnostic indicator than SUV. The cut-off level of T/N ratio for distinction between high-grade and low-grade astrocytoma appears to lie between 1.5 and 1.6.
Similar content being viewed by others
Reference
Burger PC, Shibata T, Kleihues P. The use of the monoclonal antibody Ki-67 in the identification of proliferating cells: application to surgical neuropathology.Am J Surg Pathol 1986; 10:611–617.
De Witte O, Goldberg I, Wikler D, Rorive S, Damhaut P, Monclus M, et al. Positron emission tomography with injection of methionine as a prognostic factor in glioma.J Neurosurg 2001; 95:746–750.
Dethy S, Goldman S, Blecic S, Luxen A, Levivier M, Hildebrand J. Carbon-11-methionine and fluorine- 18-FDG PET study in brain hematoma.J Nucl Med 1994; 35:1162–1166.
Di Chiro G, DeLaPaz RL, Brooks RA, Sokolff L, Kornblith PL, Smith BH, et al. Glucose utilization of cerebral gliomas measured by [18F]fluorodeoxyglucose and positron emission tomography.Neurology 1982; 32:1323–1329.
Ericson K, Blomqvist G, Bergstrom M, Eriksson L, Stone-Elander S. Application of a kinetic model on the methionine accumulation in intracranial tumours studied with positron emission tomography.Ada Radiol 1987; 28:505–509.
Ericson K, Lilja A, Bergstrom M, Collins VP, Eriksson L, Ehrin E, et al. Positron emission tomography with ([11C]methyl)-L-methionine, [11C]D-glucose, and [68Ga]EDTA in supratentorial tumors.J Comput Assist Tomogr 1985; 9:683–689.
Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67.J Immunol 1984; 133:1710–1715.
Giangaspero F, Doglioni C, Rivano MT, Pileri S, Gerdes J, Stein H. Growth fraction in human brain tumors defined by the monoclonal antibody Ki-67.Acta Neuropathol (Berl) 1987; 74:179–182.
Ishii K, Ogawa T, Hatazawa J, Kanno I, Inugami A, Fujita H, et al. High L-methyl-[11C]methionine uptake in brain abscess: a PET study.J Comput Assist Tomogr 1993; 17:660–661.
Karamitopoulou E, Perentes E, Diamantis I, Maraziotis T. Ki-67 immunoreactivity in human central nervous system tumors: a study with MIB 1 monoclonal antibody on archival material.Acta Neuropathol (Berl) 1994; 87:47–54.
Kleihues P, Burger PC, Scheithauer BW. The new WHO classification of brain tumours.Brain Pathol 1993; 3:255–268.
Lilja A, Bergstrom K, Hartvig P, Spannare B, Halldin C, Lundqvist H, et al. Dynamic study of supratentorial gliomas withl-methyl-11C-methionine and positron emission tomography.AJNR Am J Neuroradiol 1985; 6:505–514.
Ogawa T, Hatazawa J, Inugami A, Murakami M, Fujita H, Shimosegawa E, et al. Carbon-11 -methionine PET evaluation of intracerebral hematoma: distinguishing neoplastic from non-neoplastic hematoma.J Nucl Med 1995; 36:2175–2179.
Ogawa T, Kanno I, Shishido F, Inugami A, Higano S, Fujita H, et al. Clinical value of PET withl8F-fluorodeoxyglucose andl.-methyl-11C-methionine for diagnosis of recurrent brain tumor and radiation injury.Acta Radiol 1991; 32:197–202.
Patronas NJ, Di Chiro G, Kufta C, Bairamian D, Kornblith PL, Simon R, et al. Prediction of survival in glioma patients by means of positron emission tomography.J Neurosurg 1985; 62:816–822.
Patsouris E, Stocker U, Kallmeyer V, Keiditsch E, Mehraein P, Stavrou D. Relationship between Ki-67 positive cells, growth rate and histological type of human intracranial tumors.Anticancer Res 1988; 8:537–544.
Raghavan R, Steart PV, Weller RO. Cell proliferation patterns in the diagnosis of astrocytomas, anaplastic astrocytomas and glioblastoma multiforme: a Ki-67 study.Neuropathol Appl Neurobiol 1990; 16:123–133.
Schroder R, Bien K, Kott R, Meyers I, Vossing R. The relationship between Ki-67 labeling and mitotic index in gliomas and meningiomas: demonstration of the variability of the intermitotic cycle time.Acta Neuropathol (Berl) 1991; 82:389–394.
Sunada I, Tsuyuguchi N, Hara M, Ochi H.18F-FDG and11C-methionine PET in choroid plexus papilloma—report of three cases.Radiat Med 2002; 20:97–100.
Tsuyuguchi N, Ohata K, Morino M, Takami T, Goto T, Nishio A, et al. Magnetic resonance imaging and [11C] methyl-l-methionine positron emission tomography of fibrous dysplasia—two case reports.Neurol Med Chir (Tokyo) 2002; 42:341–345.
Tsuyuguchi N, Sunada I, Iwai Y, Yamanaka K, Tanaka K, Takami T. Methionine positron emission tomography of recurrent metastatic brain tumor and radiation necrosis after stereotactic radiosurgery: is a differential diagnosis possible?J Neurosurg 2003; 98:1056–1064.
Tsuyuguchi N, Takami T, Sunada I, Iwai Y, Yamanaka K, Tanaka K, et al. Methionine positron emission tomography for differentiation of recurrent brain tumor and radiation necrosis after stereotactic radiosurgery—in malignant glioma.Ann Nucl Med 2004; 18:291–296.
Tsuyuguchi N, Sunada I, Ohata K, Takami T, Nishio A, Hara M, et al. Evaluation of treatment effects in brain abscess with positron emission tomography: comparison of fluorine-18-fluorodeoxyglucose and carbon-11-methionine.Ann Nucl Med 2003; 17:47–51.
Zuber P, Hamou MF, Tribolet N. Identification of proliferating cells in human gliomas using the monoclonal antibody Ki-67.Neurosurgery 1988; 22:364–368.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Torii, K., Shiomi, S., Kawabe, J. et al. Correlation of amino-acid uptake using methionine PET and histological classifications in various gliomas. Ann Nucl Med 19, 677–683 (2005). https://doi.org/10.1007/BF02985116
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02985116