Abstract
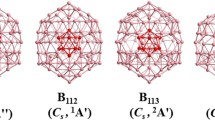
Though boranes exhibit a wide variety of polyhedral structures, all the three polymorphs of elemental boron essentially contain icosahedral B12 units as the predominant building block in their unit cell. Theoretical and experimental studies on boranes show that the icosahedral arrangement leads to most stable boranes and borane anions. This paper attempts to explain the phenomenal stability associated with the icosahedral B12 structure. Using fragment molecular orbital theory, the remarkable stability of B12H 2−12 amongcloso boranes are explained. The preferential selection icosahedral B12 unit by elemental boron is explained by improvising a contrived B84 sub-unit of the β-rhombohedron, the most stable polymorph. This also leads to a novel covalent way of stuffing fullerenes with icosahedral symmetry.
Similar content being viewed by others
References
Fleming I 1976Frontier orbitals and organic chemical reactions (New York: Wiley Inter Science)
Fox M A and Wade K 1998 inThe borane, carborane, carbocation continuum: Part II (ed.) J Casanova (New York: John-Wiley and Sons Inc.) ch. 2
Fukui K 1971Acc. Chem. Res. 4 272
Grimes R N 1970Carboranes (New York: Academic Press)
Hughes R E, Kennard C H L, Sullenger D H, Weakliem H A, Esands E E and Hoard J C 1963J. Am. Chem. Soc. 82 4427
Jemmis E D 1982J. Am. Chem. Soc. 104 7017
Jemmis E D and Kiran B 1996 inComputational chemistry—Reviews of current trends (ed.) J Leszczynski (London: World Scientific Publishing Co. Pvt. Ltd) Vol. 1, ch. 5
Jemmis E D and Kiran B 1994Curr. Sci. 66 766
Jemmis E D and Schleyer P v R 1982J. Am. Chem. Soc. 104 4781
Jemmis E D, Subramanian G and Radom L 1992J. Am. Chem. Soc. 114 1481
Jemmis E D and van Kumar P N V 1984Proc. Indian Acad. Sci. (Chem. Sci.) 93 479
Kroto H W, Heath J, O’Brien S C, Curl R F and Smalley R E 1985Nature (London) 242 1017
Libscomb W N 1963Boron hydrides (New York: Benjamin)
Muetterties E L 1975Boron hydride chemistry (New York: Academic Press)
Schleyer P v R 1998 inThe borane, carborane, carbocation continuum: Part II (ed.) J Casanova (New York: John-Wiley and Sons Inc.) ch. 7
Wells A F 1979Structural inorganic chemistry (London: Oxford University Press) ch. 24
Williams R L 1998 inThe borane, carborane, carbocation continuum: Part II (ed.) J Casanova (New York: John-Wiley and Sons Inc.) ch. 1
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jemmis, E.D., Balakrishnarajan, M.M. The ubiquitous icosahedral B12 in boron chemistry. Bull Mater Sci 22, 863–867 (1999). https://doi.org/10.1007/BF02745545
Issue Date:
DOI: https://doi.org/10.1007/BF02745545