Abstract
Background: Over one third of patients with stage II colonic adenocarcinoma experience tumor recurrence. Because effective adjuvant therapy is now available, it is important to identify subsets of patients at higher risk for relapse who may benefit from early treatment. Immunohistochemistry has been used to detect microscopic metastases in histologically uninvolved mesenteric lymph nodes, but the prognostic significance of minimal nodal involvement has not been established.
Methods: Hematoxylin and eosin (H&E)-stained recuts of 900 mesenteric lymph nodes from 55 patients (range, 2–47; mean, 16.4 nodes per case) with resected pT3 or pT4, N0, M0 (TNM stage II) colonic adenocarcinomas were re-examined for the presence of metastases and then stained immunohistochemically for keratin using the AE1:AE3 antibody. Twenty-seven patients did not experience recurrence of tumor within 5 years following resection (no evidence of disease [NED]); 28 patients relapsed during the same time frame. Lymph nodes from 10 patients having colonic resections for nonneoplastic disorders also were stained as controls. Keratin-positive cells and cell clusters were quantified in the lymph nodes, and comparisons were made between patients with and without tumor relapse.
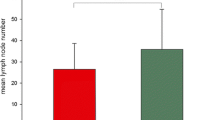
Results: In the relapse group, four patients had positive nodes already identified on the H&E-stained recuts and had to be excluded from further analysis. Sixteen additional patients had keratin-positive cells; thus, 16 of 24 (67%) had micrometastases. In the NED group, one patient had a positive node on H&E staining and 22 additional patients had keratin-positive cells, so 22 of 26 (84%) patients had micrometastases. In the patients who had micrometastases, there was a mean of 3.5 and 4.6 positive nodes in the relapse and NED groups, respectively, and a mean of 11.3 and 12.4 keratin-positive cells or clusters in the relapse and NED groups, respectively. No keratin-positive cells were found in the 1 to 21 (mean, 9.1) nodes per case studied in the control patients.
Conclusions: Micrometastases to histologically uninvolved mesenteric lymph nodes commonly are detected in patients with pT3 or pT4 colonic adenocarcinomas on recuts stained immunohistochemically for keratin. Nodal micrometastases detected by immunohistochemical staining are not useful for identifying stage II patients at higher risk for relapse.
Similar content being viewed by others
References
Cohen AM, Minsky BD, Schilsky RL. Cancer of the colon. In: DeVita VT, Hellman S, Rosenberg SA.Cancer: Principles and Practice of Oncology, 5th ed. New York: Lippincott-Raven, 1997:1144–96.
Eisenberg B, Decosse JJ, Harford F, Michalek J. Carcinoma of the colon and rectum: the natural history reviewed in 1704 patients.Cancer 1982;49:1131–4.
Adell G, Boeryd B, Franlund B, Sjodahi R, Hakansson L. Occurrence and prognostic importance of micrometastases in regional lymph nodes in Dukes’ B colorectal carcinoma: an immunohistochemical study.Eur J Surg 1996;162:637–42.
Cutait R, Alves VAF, Lopes LC, et al. Restaging of colorectal cancer based on the identification of lymph node micrometastases through immunoperoxidase staining of CEA and cytokeratins.Dis Colon Rectum 1991;34:917–20.
Greenson JK, Isenhart CE, Rice R, Mojzisik C, Houchens D, Martin EW. Identification of occult micrometastases in pericolic lymph nodes of Dukes’ B colorectal cancer patients using monoclonal antibodies against cytokeratin and CC49.Cancer 1994;73:563–9.
Haboubi NY, Clark P, Kaftan SM, Schofield PF. The importance of combining xylene clearance and immunohistochemistry in the accurate staging of colorectal carcinoma.J R Soc Med 1992;85:386–8.
Jeffers MD, O’Dowd GM, Mulcahy H, Stagg M, O’Donoghue DP, Toner M. The prognostic significance of immunohistochemically detected lymph node micrometastases in colorectal carcinoma.J Pathol 1994;172:183–7.
Makin CA, Bobrow LG, Nicholls RJ. Can immunohistology improve detection of lymph node metastases in large bowel cancer?Dis Colon Rectum 1989;32:99–102.
Broll R, Schauer V, Schimmelpennig H, et al. Prognostic relevance of occult tumor cells in lymph nodes of colorectal carcinomas.Dis Col Rectum 1997;40:1465–71.
Franke WW, Moll R. Cytoskeletal components of lymphoid organs.Differentiation 1987;36:145–63.
Iuzzolino P, Bontempini L. Keratin immunoreactivity in extrafollicular reticular cells of the lymph node. Correspondence and Corrections.Am J Clin Pathol 1989;91:239–40.
Kovarik J, Rejthar A, Lauerova L, Vojtesek B, Bartkova J. Monoclonal antibodies against individual cytokeratins in the detection of metastatic spread.Int J Cancer 1988; 3(Suppl):50–5.
Pickren JW. Significance of occult metastases. A study of breast cancer.Cancer 1961;14:1266–71.
Crowson M, Hockey MS, Newman J, Stokes H, Macdonald F, Fielding JW. An immunocytochemical study of carcinoembryonic antigen (CEA) expression in colorectal tumours and their metastases using a monoclonal antibody.Br J Surg 1984;71:376.
Davidson BR, Sams VR, Styles I, Deane C, Boulos PB. Detection of occult nodal metastases in patients with colorectal carcinoma.Cancer 1990;65:967–70.
Nakopoulou L, Zinozi M, Theodoropoulos G, Papacharalampous N. Carcinoembryonic antigen detection by immunocytochemical methods in carcinoma of the colon and stomach.Dis Colon Rectum 1983;26:269–74.
O’Brien MI, Zamcheck N, Burke B, Kirkham SE, Saravis CA, Gottlieb LS. Immunocytochemical localization of carcinoembryonic antigen in benign and malignant colorectal tissues.Am J Clin Pathol 1981;75:283–90.
Listrom MB, Dalton LW. Comparison of keratin monoclonal antibodies MAK-6, AE1:AE3, and CAM 5.2.Am J Clin Pathol 1987;88:297–301.
Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells.Cell 1982;31:11–24.
Schlimok G, Funke I, Bock B, Schweiberer B, Witte I, Riethmuller G. Epithelial tumor cells in bone marrow of patients with colorectal cancer: immunocytochemical detection, phenotypic characterization, and prognostic significance.J Clin Oncol 1990;8:831–7.
Brodsky IT, Richard GK, Cohen AM, Minsky BD. Variables correlated with the risk of lymph node metastasis in early rectal cancer.Cancer 1992;69:322–26.
Chapuis PH, Dent OF, Fisher R, Newland RC, Pheils MT, Smyth E, Colquhoun K. A multivariate analysis of clinical and pathological variables in prognosis after resection of large bowel cancer.Br J Surg 1985;72:698–702.
Fielding LP, Fenoglio-Preiser CM, Freedman LS. The future of prognostic factors in outcome prediction for patients with cancer.Cancer 1992;70:2367–77.
Cawthorn SI, Gibbs NM, Marks CG. Clearance technique for the detection of lymph nodes in colorectal cancer.Br J Surg 1986;73:58–60.
Hase K, Kochizuki H, Koike M, et al. A study on prognostic value of tumor budding in patients with rectal cancer.Jpn J Gastroenterol Surg 1992;25:2765–72.
Takizawa K. Histopathological study of rectal cancer with special reference to tumor histology and cellular stromal reaction.J Jpn Soc Coloproctol 1989;42:190–201.
Liefers GJ, Cleton-Jansen AM, VandeVelde CJH, Hermans J, van Krieken HJM, Cornelisse CJ, Tollenaar R. Micrometastases and survival in stage II colorectal cancer.N Engl J Med 1998;339:223–8.
Hayashi N, Ito I, Yanagisawa A, Kato Y, Nakamori S, Imaoka S, Watanabe H, Ogawa M, Nakamura Y. Genetic diagnosis of lymphnode metastasis in colorectal cancer.Lancet 1995;345:1257–9; commentary, p. 1255.
Burchill SA, Bradbury MF, Pittman K, Southgate I, Smith B, Selby P. Detection of epithelial cancer cells in peripheral blood by reverse transcriptase-polymerase chain reaction.Br J Cancer 1995;71:278–81.
Fidler IJ, Radinsky R. Genetic control of cancer metastasis.J Natl Cancer Inst 1990;82:166–8.
Kerbel RS. Towards an understanding of the molecular basis of the metastatic phenotype.Invasion Metastasis 1989;9:329–33.
Radinsky R. Growth factors and their receptors in metastasis.Semin Cancer Biol 1991;2:169–77.
Wright IA, Egan SE, Greenberg AH. Genetic regulation of metastatic progression.AntiCancer Res 1990;10:1247–56.
Fisher ER, Turnbull RB. The cytologic demonstration and significance of tumour cells in the mesenteric venous blood in patients with colorectal carcinoma.Surg Gynecol Obstet 1955;100:102.
Griffiths ID, McKinna IA, Rowbotham HD, Tsolakidis P, Salsbury AL. Carcinoma of the colon and rectum: circulating malignant cells and five-year survival.Cancer 1973;31:226–36.
Salsbury AL, McKinna IA, Griffiths ID, Morgan CN. Circulating cancer cells during excision of carcinoma of the rectum and colon with high ligation of the inferior mesenteric vein.Surg Gynecol Obstet 1965;120:1266.
Kitadai Y, Bucana CD, Ellis LM, Anzai H, Tahara E, Fidler IJ. In situ mRNA hybridization technique for analysis of metastasis-related genes in human colon carcinoma cells.Am J Pathol 1995;147:1238–47.
Liotta LA, Stetler-Stevenson WG. Tumor invasion and metastasis: an imbalance of positive and negative regulation.Cancer Res 1991;51(Suppl):5054–9.
Radinsky R, Risin S, Fan D, Dong Z, Bielenberg D, Bucana CD, Fidler IJ. Level and function of epidermal growth factor receptor predict the metastatic potential of human colon carcinoma cells.Clin Cancer Res 1995;1:19–31.
Oberg A, Stenling R, Tavelin B, Lindmark G. Are lymph node micrometastases of any clinical significance in Dukes stages A and B colorectal cancer?Dis Colon Rectum 1998;41:1244–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tschmelitsch, J., Klimstra, D.S. & Cohen, A.M. Lymph node micrometastases do not predict relapse in stage II colon cancer. Ann Surg Oncol 7, 601–608 (2000). https://doi.org/10.1007/BF02725340
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02725340