Abstract
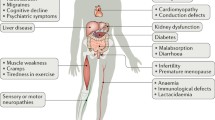
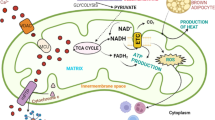
The pathophysiology of mitochondrial DNA (mtDNA) diseases is caused by increased cell death and dysfunction due to the accumulation of mutations to mtDNA. While the disruption of oxidative phosphorylation is central to mtDNA diseases, many other factors, such as Ca2+ dyshomeostasis, increased oxidative stress and defective turnover of mitochondrial proteins, may also contribute. The relative importance of these processes in causing cell dysfunction and death is uncertain. It is also unclear whether these damaging processes lead to the disease phenotype through affecting cell function, increasing cell death or a combination of both. These uncertainties limit our understanding of mtDNA disease pathophysiology and our ability to develop rational therapies. Here, we outline how the accumulation of mtDNA mutations can lead to cell dysfunction by altering oxidative phosphorylation, Ca2+ homeostasis, oxidative stress and protein turnover and discuss how these processes affect cell function and susceptibility to cell death. A better understanding of these processes will eventually clarify why particular mtDNA mutations cause defined syndromes in some cases but not in others and why the same mutation can lead to different phenotypes.
Similar content being viewed by others
References
Adams JM, Cory S. Life-or-death decisions by the Bcl-2 protein family. Trends Biochem Sci 26:61–66;2001.
Albin RL, Greenamyre JT. Alternative excitotoxic hypotheses. Neurology 42:733–738;1992.
Anderson S, Bankier AT, Barrell BG, de Bruijn MHL, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJH, Staden R, Young IG. Sequence and organization of the human mitochondrial genome. Nature 290:457–465;1981.
Asoh S, Mori T, Hayashi J, Ohta S. Expression of the apoptosis-mediator Fas is enhanced by dysfunctional mitochondria. J Biochem (Tokyo) 120:600–607;1996.
Azzi A, Montecucco C, Richter C. The use of acetylated ferricytochrome c for the detection of superoxide radicals produced in biological membranes. Biochem Biophys Res Commun 65:597–603;1975.
Baker SK, Tarnopolsky MA, Bonen A. Expression of MCT1 and MCT4 in a patient with mitochondrial myopathy. Muscle Nerve 24:394–398;2001.
Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am J Physiol 271:C1424-C1437;1996.
Bernardi P, Petronilli V, Di Lisa F, Forte M. A mitochondrial perspective on cell death. Trends Biochem Sci 26:112–117;2001.
Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1:11–21;2000.
Boulet L, Karpati G, Shoubridge EA. Distribution and threshold expression of the tRNA-(Lys) mutation in skeletal muscle of patients with myoclonic epilepsy and ragged-red fibers (MERRF). Am J Hum Genet 51:1187–1200;1992.
Brini M, Pinton P, King MP, Davidson M, Schon EA, Rizzuto R. A calcium signaling defect in the pathogenesis of a mitochondrial DNA inherited oxidative phosphorylation deficiency. Nat Med 5:951–954;1999.
Casari G, De Fusco M, Ciarmatori S, Zeviani M, Mora M, Fernandez P, De Michele G, Filla A, Cocozza S, Marconi R, Durr A, Fontaine B, Ballabio A. Spastic paraplegia and OXPHOS impairment caused by mutations in paraplegin, a nuclear-encoded mitochondrial metalloprotease. Cell 93:973–983;1998.
Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev 59:527–605;1979.
Chen Z, Siu B, Ho YS, Vincent R, Chua CC, Hamdy RC, Chua BH. Overexpression of MnSOD protects against myocardial ischemia/reperfusion injury in transgenic mice. J Mol Cell Cardiol 30:2281–2289;1998.
Chernyak BV, Bernardi P. The mitochondrial permeability transition pore is modulated by oxidative agents through both pyridine nucleotides and glutathione at two separate sites. Eur J Biochem 238:623–630;1996.
Chinnery PF, Thorburn DR, Samuels DC, White SL, Dahl HM, Turnbull DM, Lightowlers RN, Howell N. The inheritance of mitochondrial DNA heteroplasmy: Random drift, selection or both? Trends Genet 16:500–505;2000.
Cottrell DA, Ince PG, Blakely EL, Johnson MA, Chinnery PF, Hanna M, Turnbull DM. Neuropathological and histochemical changes in a multiple mitochondrial DNA deletion disorder. J Neuropathol Exp Neurol 59:621–627;2000.
Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J 341:233–249;1999.
Dawson G, Cho S. Batten's disease: Clues to neuronal protein catabolism in lysosomes. J Neurosci Res 60:133–140;2000.
Delgado-Escueta AV, Ganesh S, Yamakawa K. Advances in the genetics of progressive myoclonus epilepsy. Am J Med Genet 106:129–138;2001.
Demaurex N, Grinstein S. Na+/H+ antiport: Modulation by ATP and role in cell volume regulation. J Exp Biol 196:389–404;1994.
Dey R, Moraes CT. Lack of oxidative phosphorylation and low mitochondrial membrane potential decrease susceptibility to apoptosis and do not modulate the protective effect of Bcl-x(L) in osteosarcoma cells. J Biol Chem 275:7087–7094;2000.
Di Giovanni S, Mirabella M, Papacci M, Odoardi F, Silvestri G, Servidei S. Apoptosis and ROS detoxification enzymes correlate with cytochrome c oxidase deficiency in mitochondrial encephalomyopathies. Mol Cell Neurosci 17:696–705;2001.
DiMauro S, Hirano M, Schon EA. Mitochondrial encephalomyopathies: Therapeutic approaches. Neurol Sci 21(5 suppl):S901-S908;2000.
DiMauro S, Schon EA. Mitochondrial DNA mutations in human disease. Am J Med Genet 106:18–26;2001.
Drachev LA, Kondrashin AA, Semenov AY, Skulachev VP. Reconstitution of biological molecular generators of electric current. Transhydrogenase. Eur J Biochem 113:213–217;1980.
Duchen MR. Mitochondria and calcium: From cell signalling to cell death. J Physiol 529:57–68;2000.
Elmore SP, Qian T, Grissom SF, Lemasters JJ. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J 15:2286–2287;2001.
Enriquez JA, Chomyn A, Attardi G. MtDNA mutation in MERRF syndrome causes defective aminoacylation of tRNA(Lys) and premature translation termination. Nat Genet 10:47–55;1995.
Ezaki J, Wolfe LS, Higuti T, Ishidoh K, Kominami E. Specific delay of degradation of mitochondrial ATP synthase subunit c in late infantile neuronal ceroid lipofuscinosis (Batten disease). J Neurochem 64:733–741;1995.
Forsmark P, Aberg F, Norling B, Nordenbrand K, Dallner G, Ernster L. Inhibition of lipid peroxidation by ubiquinol in submitochondrial particles in the absence of vitamin E. FEBS Lett 285:39–43;1991.
Geromel V, Kadhom N, Cebalos-Picot I, Ouari O, Polidori A, Munnich A, Rotig A, Rustin P. Superoxide-induced massive apoptosis in cultured skin fibroblasts harboring the neurogenic ataxia retinitis pigmentosa (NARP) mutation in the ATPase-6 gene of the mitochondrial DNA. Hum Mol Genet 10:1221–1228;2001.
Goldstone TP, Roos I, Crompton M. Effects of adrenergic agonists and mitochondrial energy state on the Ca2+ transport systems of mitochondria. Biochemistry 26:246–254;1987.
Goto Y. Clinical and molecular studies of mitochondrial disease. J Inherit Metab Dis 24:181–188;2001.
Gunter TE, Buntinas L, Sparagna G, Eliseev R, Gunter K. Mitochondrial calcium transport: Mechanisms and functions. Cell Calcium 28:285–296;2000.
Gutteridge JM, Halliwell B. Free radicals and antioxidants in the year 2000. A historical look to the future. Ann NY Acad Sci 899:136–147;2000.
Hansford RG, Zorov D. Role of mitochondrial calcium transport in the control of substrate oxidation. Mol Cell Biochem 184:359–369;1998.
Harris MH, Thompson CB. The role of the Bcl-2 family in the regulation of outer mitochondrial membrane permeability. Cell Death Differ 7:1182–1191;2000.
Heddi A, Lestienne P, Wallace DC, Stepien G. Mitochondrial DNA expression in mitochondrial myopathies and coordinated expression of nuclear genes involved in ATP production. J Biol Chem 268:12156–12163;1993.
Heddi A, Stepien G, Benke PJ, Wallace DC. Coordinate induction of energy gene expression in tissues of mitochondrial disease patients. J Biol Chem 274:22968–22976;1999.
Henneberry RL, Novelli A, Lysko PG. Neurotoxicity at the N-methyl-D-aspartate receptor in energy-compromised neurons. An hypothesis for cell death in aging and disease. Ann NY Acad Sci 568:225–233;1989.
Higuchi M, Aggarwal BB, Yeh ET. Activation of CPP32-like protease in tumor necrosis factor-induced apoptosis is dependent on mitochondrial function. J Clin Invest 99:1751–1758;1997.
Holtzman E: Lysosomes, ed 1. New York, Plenum, 1989.
Hwang PM, Bunz F, Yu J, Rago C, Chan TA, Murphy MP, Kelso GF, Smith RA, Kinzler KW, Vogelstein B. Ferredoxin reductase affects p53-dependent, 5-fluorouracil-induced apoptosis in colorectal cancer cells. Nat Med 7:1111–1117;2001.
Inoue K, Nakada K, Ogura A, Isobe K, Goto Y, Nonaka I, Hayashi JI. Generation of mice with mitochondrial dysfunction by introducing mouse mtDNA carrying a deletion into zygotes. Nat Genet 26:176–181;2000.
Ishikawa Y, Asuwa N, Ishii T, Masuda S, Kiguchi H, Hirai S, Akashi N, Yonenami K, Fujisawa Y. Severe mitochondrial cardiomyopathy and extra-neuromuscular abnormalities in mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episode (MELAS). Pathol Res Pract 191:64–75;1995.
Jacobson MD, Burne JF, King MP, Miyashita T, Reed JC, Raff MC. Bcl-2 blocks apoptosis in cells lacking mitochondrial DNA. Nature 361:365–369;1993.
James AJ, Sheard PW, Wei Y-H, Murphy MP. Decreased ATP synthesis is phenotypically expressed during increased energy demand in fibroblasts containing mitochondrial tRNA mutations: Implications for neurodegenerative and mitochondrial DNA diseases. Eur J Biochem 259:462–469;1999.
James AM, Wei Y-H, Pang C-Y, Murphy MP. Altered mitochondrial function in fibroblasts containing MELAS or MERRF mitochondrial DNA mutations. Biochem J 318:401–407;1996.
Jiang S, Cai J, Wallace DC, Jones DP. Cytochrome c-mediated apoptosis in cells lacking mitochondrial DNA. Signaling pathway involving release and caspase 3 activation is conserved. J Biol Chem 274:29905–29911;1999.
Kalman B, Lublin FD, Alder H. Impairment of central and peripheral myelin in mitochondrial diseases. Mult Scler 2:267–278;1997.
Kawai H, Akaike M, Yokoi K, Nishida Y, Kunishige M, Mine H, Saito S. Mitochondrial encephalomyopathy with autosomal dominant inheritance: A clinical and genetic entity of mitochondrial diseases. Muscle Nerve 18:753–760;1995.
Kegel KB, Kim M, Sapp E, McIntyre C, Castano JG, Aronin N, DiFiglia M. Huntingtin expression stimulates endosomal-lysosomal activity, endosome tubulation, and autophagy. J Neurosci 20:7268–7278;2000.
Keller JN, Kindy MS, Holtsberg FW, St Clair DK, Yen HC, Germeyer A, Steiner SM, Bruce-Keller AJ, Hutchins JB, Mattson MP. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: Suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J Neurosci 18:687–697;1998.
Kerrison JB, Howell N, Miller NR, Hirst L, Green WR. Leber hereditary optic neuropathy. Electron microscopy and molecular genetic analysis of a case. Ophthalmology 102:1509–1516;1995.
King MP, Koga Y, Davidson M, Schon EA. Defects in mitochondrial protein synthesis and respiratory chain activity segregate with the tRNALeu(UUR) mutation associated with mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes. Mol Cell Biol 12:480–490;1992.
Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science 290:1717–1721;2000.
Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett 416:15–18;1997.
Larsson NG, Tulinius MH, Holme E, Oldfors A, Andersen O, Wahlstrom J, Aasly J. Segregation and manifestations of the mtDNA tRNA-(Lys) A->G(8344) mutation of myoclonus epilepsy and ragged-red fibers (MERRF) syndrome. Am J Hum Genet 51:1201–1212;1992.
Larsson NG, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, Barsh GS, Clayton DA. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet 18:231–236;1998.
Li H, Wang J, Wilhelmsson H, Hansson A, Thoren P, Duffy J, Rustin P, Larsson NG. Genetic modification of survival in tissue-specific knockout mice with mitochondrial cardiomyopathy. Proc Natl Acad Sci USA 97:3467–3472;2000.
Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402:672–676;1999.
Lombes A, Mendell JR, Nakase H, Barohn RJ, Bonilla E, Zeviani M, Yates AJ, Omerza J, Gales TL, Nakahara K, et al. Myoclonic epilepsy and ragged-red fibers with cytochrome oxidase deficiency: Neuropathology, biochemistry, and molecular genetics. Ann Neurol 26:20–33;1989.
Luo XP, Pitkänen S, Kassovska-Bratinova S, Robinson BH, Lehotay DC. Excessive formation of hydroxyl radicals and aldehydic lipid perioxidation products in cultured skin fibroblasts from patients with complex I deficiency. J Clin Invest 99:2877–2882;1997.
Macmillan-Crow LA, Cruthirds DL. Invited review: Manganese superoxide dismutase in disease. Free Radic Res 34:325–336;2001.
Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature 309:261–263;1984.
Miller RJ, Murphy SN, Glaum SR. Neuronal Ca2+ channels and their regulation by excitatory amino acids. Ann NY Acad Sci 568:149–158;1989.
Mirabella M, Di Giovanni S, Silvestri G, Tonali P, Servidei S. Apoptosis in mitochondrial encephalomyopathies with mitochondrial DNA mutations: A potential pathogenic mechanism. Brain 123:93–104;2000.
Mita S, Tokunaga M, Kumamoto T, Uchino M, Nonaka I, Ando M. Mitochondrial DNA mutation and muscle pathology in mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes. Muscle Nerve Suppl 3:S113-S118;1995.
Mita S, Tokunaga M, Uyama E, Kumamoto T, Uekawa K, Uchino M. Single muscle fiber analysis of myoclonus epilepsy with ragged-red fibers. Muscle Nerve 21:490–497;1998.
Monici MC, Toscano A, Girlanda P, Aguennouz M, Musumeci O, Vita G. Apoptosis in metabolic myopathies. Neuroreport 9:2431–2435;1998.
Moraes CT. Mitochondrial disorders. Curr Opin Neurol 9:369–374;1996.
Moudy AM, Handran SD, Goldberg MP, Ruffin N, Karl I, Kranz-Eble P, DeVivo DC, Rothman SM. Abnormal calcium homeostasis and mitochondrial polarization in a human encephalomyopathy. Proc Natl Acad Sci USA 92:729–733;1995.
Murphy MP. Development of lipophilic cations as therapies for disorders due to mitochondrial dysfunction. Expert Opin Biol Ther 1:753–764;2001.
Murphy MP, Packer MA, Scarlett JL, Martin SW, Peroxynitrite: A biologically significant oxidant. Gen Pharmacol 31:179–186;1998.
Newman NJ. Leber's hereditary optic neuropathy. New genetic considerations. Arch Neurol 50:540–548;1993.
Nixon RA, Cataldo AM, Mathews PM. The endosomal-lysosomal system of neurons in Alzheimer's disease pathogenesis: A review. Neurochem Res 25:1161–1172;2000.
Pajic A, Tauer R, Feldmann H, Neupert W, Langer T. Yta10p is required for the ATP-dependent degradation of polypeptides in the inner membrane of mitochondria. FEBS Lett 353:201–206;1994.
Palmer DN, Bayliss SL, Westlake VJ. Batten disease and the ATP synthase subunit C turnover pathway. Am J Med Genet 57:260–265;1995.
Paul M-F, Tzagoloff A. Mutations inRCA1 andAFG3 inhibit F1-ATPase assembly inSaccharomyces cerevisiae. FEBS Lett 373:66–70;1995.
Pavlakis SG, Phillips PC, DiMauro S, De Vivo DC, Rowland LP. Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes: A distinctive clinical syndrome. Ann Neurol 16:481–488;1984.
Pitkänen S, Robinson BH. Mitochondrial complex I deficiency leads to increased production of superoxide radicals and induction of superoxide dismutase. J Clin Invest 98:345–351;1996.
Plum F. What causes infarction in ischemic brain? The Robert Wartenberg lecture. Neurology 33:222–233;1983.
Porteous WK, James AM, Sheard PW, Porteous CM, Packer MA, Hyslop SJ, Melton JV, Pang CY, Wei YH, Murphy MP. Bioenergetic consequences of accumulating the common 4977-bp mitochondrial DNA deletion. Eur J Biochem 257:192–201;1998.
Raha S, Robinson BH. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci 25:502–508;2000.
Reichmann H. Enzyme activity analyses along ragged-red and normal single muscle fibres. Histochemistry 98:131–134;1992.
Reid FM, Vernham GA, Jacobs HT. A novel mitochondrial point mutation in a maternal pedigree with sensorineural deafness. Hum Mutat 3:243–247;1994.
Scelsi R. Morphometric analysis of skeletal muscle fibres and capillaries in mitochondrial myopathies. Pathol Res Pract 188:607–611;1992.
Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 30:1191–1212;2001.
Sciacco M, Fagiolari G, Lamperti C, Messina S, Bazzi P, Napoli L, Chiveri L, Prelle A, Comi GP, Bresolin N, Scarlato G, Moggio M. Lack of apoptosis in mitochondrial encephalomyopathies. Neurology 56:1070–1074;2001.
Sevior KB, Hatamochi A, Stewart IA, Bykhovskaya Y, Allen-Powell DR, Fischel-Ghodsian N, Maw MA. Mitochondrial A7445G mutation in two pedigrees with palmoplantar keratoderma and deafness. Am J Med Genet 75:179–185;1998.
Siesjö BK, Bengtsson F, Grampp W, Theander S. Calcium, excitotoxins, and neuronal death in the brain. Ann NY Acad Sci 568:234–251;1989.
Silva JP, Kohler M, Graff C, Oldfors A, Magnuson MA, Berggren PO, Larsson NG. Impaired insulin secretion and beta-cell loss in tissue-specific knockout mice with mitochondrial diabetes. Nat Genet 26:336–340;2000.
Smeitink J, van den Heuvel L, DiMauro S. The genetics and pathology of oxidative phosphorylation. Nat Rev Genet 2:342–352;2001.
Sorensen L, Ekstrand M, Silva JP, Lindqvist E, Xu B, Rustin P, Olson L, Larsson NG. Lateonset corticohippocampal neurodepletion attributable to catastrophic failure of oxidative phosphorylation in MILON mice. J Neurosci 21:8082–8090;2001.
Sparaco M, Bonilla E, DiMauro S, Powers JM. Neuropathology of mitochondrial encephalomyopathies due to mitochondrial DNA defects. J Neuropathol Exp Neurol 52:1–10;1993.
Tauer R, Mannhaupt G, Schnall R, Pajic A, Langer T, Feldmann H. Yta10p, a member of a novel ATPase family in yeast, is essential for mitochondrial function. FEBS Lett 353:197–200;1994.
Terauchi A, Tamagawa K, Morimatsu Y, Kobayashi M, Sano T, Yoda S. An autopsy case of mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS) with a point mutation of mitochondrial DNA. Brain Dev 18:224–229;1996.
Terman A. Garbage catastrophe theory of aging: Imperfect removal of oxidative damage? Redox Rep 6:15–26;2001.
Tomkinson B. Tripeptidyl peptidases: Enzymes that count. Trends Biochem Sci 24:355–359;1999.
Tzagoloff A, Yue J, Jang J, Paul MF. A new member of a family of ATPases is essential for assembly of the mitochondrial respiratory chain and ATP synthetase complexes inSaccharomyces cerevisiae. J Biol Chem 269:26144–26151;1994.
Wang J, Silva JP, Gustafsson CM, Rustin P, Larsson NG. Increased in vivo apoptosis in cells lacking mitochondrial DNA gene expression. Proc Natl Acad Sci USA 98:4038–4043;2001.
Wang J, Wilhelmsson H, Graff C, Li H, Oldfors A, Rustin P, Bruning JC, Kahn CR, Clayton DA, Barsh GS, Thoren P, Larsson NG. Dilated cardiomyopathy and atrioventricular conduction blocks induced by heart-specific inactivation of mitochondrial DNA gene expression. Nat Genet 21:133–137;1999.
Williams AJ, Cole PJ. In vitro stimulation of alveolar macrophage metabolic activity by polystyrene in the absence of phagocytosis. Br J Exp Pathol 62:1–7;1981.
Wong A, Cortopassi G. mtDNA mutations confer cellular sensitivity to oxidant stress that is partially rescued by calcium depletion and cyclosporin A. Biochem Biophys Res Commun 239:139–145;1997.
Xue L, Fletcher GC, Tolkovsky AM. Autophagy is activated by apoptotic signalling in sympathetic neurons: An alternative mechanism of death execution. Mol Cell Neurosci 14:180–198;1999.
Yasukawa T, Suzuki T, Ishii N, Ohta S, Watanabe K. Wobble modification defect in tRNA disturbs codon-anticodon interaction in a mitochondrial disease. EMBO J 20:4794–4802;2001.
Zeviani M, Corona P, Nijtmans L, Tiranti V. Nuclear gene defects in mitochondrial disorders. Ital J Neurol Sci 20:401–408;1999.
Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim Biophys Acta 1241:139–176;1995.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
James, A.M., Murphy, M.P. How mitochondrial damage affects cell function. J Biomed Sci 9, 475–487 (2002). https://doi.org/10.1007/BF02254975
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02254975