Abstract
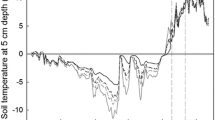
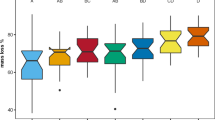
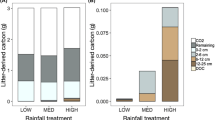
Mass and nitrogen (N) dynamics of leaf litter measured in Alaskan tussock tundra differed greatly from measurements of these processes made in temperate ecosystems. Nearly all litter mass and N loss occurred during the winter when soils were mostly frozen. Litter lost mass during the first summer, but during the subsequent two summers when biological activity was presumably higher than it is during winter, litter mass remained constant and litter immobilized N. By contrast, litter lost significant mass and N over both winters of measurement. Mass loss and N dynamics were unaffected by microsite variation in soil temperature and moisture. Whether wintertime mass and N loss resulted from biological activity during winter or from physical processes (e.g., fragmentation or leaching) associated with freeze-thaw is unknown, but has implications for how future climate warming will alter carbon (C) and N cycling in tundra. We hypothesize that spring runoff over permafrost as soils melt results in significant losses of C and N from litter, consistent with the observed influx of terrestrial organic matter to tundra lakes and streams after snow melt and the strong N limitation of terrestrial primary production.
Similar content being viewed by others
References
Bleak AT (1970) Disappearance of plant material under a winter snow cover. Ecology 51: 915–917
Busby JR, Bliss LC & Hamilton CD (1978) Microclimate control of growth rates and habitats of the boreal forest mosses,Tomenthypnum nitens andHylocomium splendens. Ecol. Monogr. 48: 95–110
Chapin FS III & Bloom A (1976) Phosphate absorption: adaptation of tundra graminoids to low temperature, low-phosphorus environment. Polar Biol. 2: 37–52
Chapin FS III, Fetcher N, Kielland K, Everett KR & Linkins AE (1988) Productivity and nutrient cycling of Alaskan tundra: Enhancement by flowing soil water. Ecology 69: 693–702
Chapin FS III & Kedrowski RA (1983) Seasonal changes in nitrogen and phosphorus fractions and autumn retranslocation in evergreen and deciduous taiga trees. Ecology 64: 376–391
Chapin FS III, Moilanen L & Kielland K (1993) Preferential use of organic nitrogen for growth by a non-mycorrhizal arctic sedge. Nature 361: 150–153
Chapin FS III & Shaver GR (1988) Differences in carbon and nutrient fractions among arctic growth forms. Oecologia 77: 506–514
Chapin FS III, Van Cleve K & Chapin MC (1979) Soil temperature and nutrient cycling in the tussock growth form ofEriophorum vaginatum. J. Ecol. 67: 169–189
Clymo RS & Hayward PM (1982) The ecology ofSphagnum. In: Smith AJE (Ed) Bryophyte Ecology (pp 229–289). Chapman and Hall, London
Coxson DS & Parkinson D (1987) Winter respiratory activity in Aspen woodland forest floor litter and soils. Soil Biol. Biochem. 19: 49–59
Edwards NT (1975) Effects of temperature and moisture on carbon dioxide evolution in a mixed deciduous forest floor. Soil Sci. Soc. Am. Proc. 39: 361–365
Flanagan PW & Veum AK (1974) Relationships between respiration, weight loss, temperature, and moisture in organic residues on tundra. In: Holding AJ, Heal OW, Maclean SF Jr. & Flanagan PW (Eds) Soil Organisms and Decomposition in Tundra (pp 249–277). Tundra Biome Steering Committee, Stockholm
Giblin AE, Laundre JA, Nadelhoffer KJ & Shaver GR (1994) Measuring nutrient availability in arctic soils using ion exchange resins: a field test. Soil Sci. Soc. Amer. J. 58: 1154–1162
Gosz JR, Likens GE & Bormann FH (1973) Nutrient release from decomposing leaf and branch litter in the Hubbard Brook forest, New Hampshire. Ecol. Monogr. 43: 173–191
Hågvar S & Kjøndal BR (1981) Decomposition of birch leaves: dry weight loss, chemical changes, and effects of artificial acid rain. Pedobiologia 22: 232–245
Heal OW, Latter PM & Howson G (1978) A study of the rates of decomposition of organic matter. In: Heal OW & Perkins DF (Eds) Production Ecology of British Moorlands and Montane Grasslands (pp 136–159). Springer-Verlag, Berlin
Hinzman LD, Kane DL, Gieck RE & Everett KR (1991) Hydrologic and thermal properties of the active layer in the Alaskan Arctic. Cold Reg. Sci. Technol. 19: 95–110
Hobbie SE (In press) Temperature and plant species control over litter decomposition in Alaskan tundra. Ecol. Monogr.
Ivarson KC & Sowden FJ (1970) Effect of frost action and storage of soil at freezing temperatures on the free amino acids, free sugars and respiratory activity of soil. Can. J. Soil Sci. 50: 191–198
Kane DL, Hinzman LD, Woo M & Everett KR (1992) Arctic hydrology and climate change. In: Chapin FS III, Jefferies RL, Reynolds JF, Shaver GR & Svoboda J (Eds) Arctic Ecosystems in a Changing Climate: An Ecophysiological Perspective (pp 35–57). Academic Press, San Diego
Kielland K (1990) Processes controlling nitrogen release and turnover in arctic tundra. PhD, University of Alaska, Fairbanks
Kling GW (1995) Land-water interactions: the influence of terrestrial diversity on aquatic ecosystems. In: Chapin FS III & Körner C (Eds) Arctic and Alpine Biodiversity: Patterns, Causes and Ecosystem Consequences (pp 297–310). Springer-Verlag, Berlin
Kucera CL & Kirkham DR (1971) Soil respiration studies in tallgrass prairie in Missouri. Ecology 52: 912–915
Lousier JD & Parkinson D (1975) Litter decomposition in a cool temperate deciduous forest. Can. J. Bot. 54: 419–436
Marion GM & Black CH (1986) The effect of time and temperature on nitrogen mineralization in arctic tundra soils. Soil Sci. Soc. Amer. J. 51: 1501–1508
Maxwell B (1992) Arctic climate: potential for change under global warming. In: Chapin FS III, Jefferies RL, Reynolds JF, Shaver GR & Svoboda J (Eds) Arctic Ecosystems in a Changing Climate: An Ecophysiological Perspective (pp 11–34). Academic Press, San Diego, California, USA
McBrayer JF & Cromack K Jr (1980) Effect of snow-pack on oak-litter breakdown and nutrient release in a Minnesota forest. Pedobiologia 20: 47–54
McKane RB, Rastetter EB, Shaver GR, Nadelhoffer KJ, Giblin AE, Laundre JA & Chapin FS III (In preparation) Analysis of the effects of climate change on carbon storage of arctic tundra
Melillo JM, Aber JD, Linkins AE, Ricca A, Fry B & Nadelhoffer KJ (1989) Carbon and nitrogen dynamics along the decay continuum: Plant litter to soil organic matter. Plant Soil 115: 189–198
Melillo JM, Aber JD & Muratore JF (1982) Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63: 621–626
Murray KJ, Harley PC, Beyers J, Walz H & Tenhunen JD (1989) Water content effects on photosynthetic response ofSphagnum mosses from the foothills of the Philip Smith Mountains, Alaska. Oecologia 79: 244–250
Nadelhoffer KJ, Giblin AE, Shaver GR & Laundre JA (1991) Effects of temperature and substrate quality on element mineralization in six arctic soils. Ecology 72: 242–253
Nadelhoffer KJ, Giblin AE, Shaver GR & Linkins AE (1992) Microbial processes and plant nutrient availability in arctic soils. In: Chapin FS III, Jefferies RL, Reynolds JF, Shaver GR & Svoboda J (Eds) Arctic Ecosystems in a Changing Climate: An Ecophysiological perspective (pp 281–300). Academic Press, San Diego
Oechel WC, Hastings SJ, Vourlitis G, Jenkins M, Riechers G & Grulke N (1993) Recent change of Arctic tundra ecosystems from a net carbon dioxide sink to a source. Nature 361: 520–523
Pastor J, Stillwell MA & Tilman D (1987) Little bluestem litter dynamics in Minnesota old fields. Oecologia 72: 327–330
Paul EA & Clark FE (1989) Soil microbiology and biochemistry. Academic Press, San Diego
Peterson BJ, Corliss TL, Kriet K & Hobbie JE (1992) Nitrogen and phosphorus concentrations and export for the upper Kuparuk River on the North Slope of Alaska in 1980. Hydrobiologia 240: 61–69
Read DJ (1991) Mycorrhizas in ecosystems. Experientia 47: 376–391
Reiners WA (1968) Carbon dioxide evolution from the floor of three Minnesota forests. Ecology 49: 471–483
Ryan MG, Melillo JM & Ricca A (1989) A comparison of methods for determining proximate carbon fractions of forest litter. Can. J. For. Res. 20: 166–171
Shaver GR & Billings WD (1975) Root production and root turnover in a wet tundra ecosystem, Barrow, Alaska. Ecology 56: 401–410
Shaver GR, Billings WD, Chapin FS III, Giblin AE, Nadelhoffer KJ, Oechel WC & Rastetter EB (1992) Global change and the carbon balance of arctic ecosystems. BioScience 61: 415–435
Shaver GR & Chapin FS III (1986) Effect of fertilizer on production and biomass of tussock tundra, Alaska, U.S.A. Arct. Alp. Res. 18: 261–268
Skogland T, Lomeland S & Goksøyr J (1988) Respiratory burst after freezing and thawing of soil: experiments with soil bacteria. Soil Biol. Biochem. 20: 851–856
Staaf H & Berg B (1981) Accumulation and release of plant nutrients in decomposing Scots pine needle litter. Long-term decomposition in a Scots pine forest II. Can. J. Bot. 60: 1561–1568
Townsend AR, Vitousek PM & Holland EA (1992) Tropical soils could dominate the short-term carbon cycle feedbacks to increased global temperatures. Climatic Change 22: 293–303
Whalen SC & Cornwell JC (1985) Nitrogen, phosphorus, and organic carbon cycling in an arctic lake. Can. J. Fish. Aquat. Sci. 42: 797–808
Wiant HV (1967) Influence of temperature on the rate of soil respiration. J. Forest. 65: 489–490
Zimov SA, Semiletov IP, Davidov SP, Voropaev IV, Prosyannikov SF, Wong CS & Chan Y-H (1993) Wintertime CO2 emission from soils of northeastern Siberia. Arctic 46: 197–204
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hobbie, S.E., Chapin, F.S. Winter regulation of tundra litter carbon and nitrogen dynamics. Biogeochemistry 35, 327–338 (1996). https://doi.org/10.1007/BF02179958
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02179958