Abstract
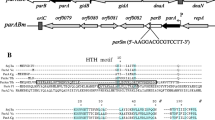
We have previously reported that the MukB protein is essential for chromosome partitioning inEscherichia coli and thatmukB mutants produce anucleate cells and are temperature-sensitive for colony formation. ThemukB gene maps at 21 min on theE. coli chromosome andsmtA-mukF-mukE-mukB genes might comprise an operon, which is transcribed in a clockwise direction. Here, we report thatmukF andmukE null mutants are both temperature-sensitive for colony formation and produce anucleate cells even at the permissive temperature. These phenotypes are the same as those observed in themukB null mutant. The primary sequence of MukF includes a leucine zipper structure and an acidic domain. Mutational analysis revealed that both are required for MukF function. When the MukF protein was overproduced in the wild-type strain, anucleate cells were produced. In contrast, overproduction of either MukE or MukB did not cause the defect. In null mutants for themukF, mukE, andmukB genes, the synchronous initiation of chromosome replication was not affected. The mini-F plasmid was as stably maintained in these mutants as in the wild-type strain. These results indicate that the MukF, MukE, and MukB proteins are involved in the chromosome partitioning steps, but are not required for mini-F plasmid partitioning.
Similar content being viewed by others
References
Altschull SF, Gish W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Baksh S, Michalak M (1991) Expression of calreticulin inEscherichia coli and identification of its Ca2+ binding domains. J Biol Chem 266:21485–21465
Chang ACY, Cohen SN (1978) Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol 134:1141–1156
Chiu E, Boyle WJ, Meek J, Smeal T, Karin M (1988) The c-fos protein interacts with a c-jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell 54:541–552
Donachie WD (1993) The cell cycle ofEscherichia coli. Annu Rev Microbiol 47:199–230
Earnshaw WC, Sullivan KF, Machlin PS, Cooke CA, Kaiser DA, Pollard TD, Rothfield NF, Cleveland DW (1987) Molecular cloning of cDNA for CENP-B, the major human centromere autoantigen. J Cell Biol 104:817–829
Ezaki B, Ogura T, Niki H, Hiraga S (1991) Partitioning of a mini-F plasmid into anucleate cells of themukB null mutant. J Bacteriol 173:6643–6646
Feng J, Yamanaka K, Niki H, Ogura T, Hiraga S (1994) New killing system controlled by two genes located immediately upstream of themukB gene inEscherichia coli. Mol Gen Genet 243:136–147
Fleischmann RD, Adams MD, White O, Clayton RA, Kirkness EF, Kerlavage AR, Bult CJ, Tomb J-F, Dougherty BA, Merrick JM, McKenney K, Sutton G, FitzHugh W, Fields C, Gocayne JD, Scott J, Shirley R, Liu L-l, Glodek A, Kelley JM, Weidman JF, Phillips CA, Spriggs T, Hedblom E, Cotton MD, Utterback TR, Hanna MC, Nguyen DT, Saudek DM, Brandon RC, Fine LD, Fritchman JL, Fuhrmann JL, Geoghagen NSM, Gnehm CL, McDonald LA, Small KV, Fraser CM, Smith HO, Venter JC (1995) Whole-genome random sequencing and assembly ofHaemophilus influenzae Rd. Science 269:496–512
Funnell BE, Gagnier L (1995) Partition of P1 plasmid inEscherichia coli mukB chromosomal partition mutants. J Bacteriol 177:2381–2386
Guzman L-M, Barondess JJ, Beckwith J (1992) FtsL, an essential cytoplasmic membrane protein involved in cell division inEscherichia coli. J Bacteriol 174: 7716–7728
Halazonetis TD, Georgopoulos K, Greenberg ME, Leder P (1988) c-Jun dimerizes with itself and with c-Fos, forming complexes of different DNA binding affinities. Cell 55:917–924
Hattori M, Sakaki Y (1986) Dideoxy sequencing method using denatured plasmid templates. Anal Biochem 152:232–238
Hiraga S (1992) Chromosome and plasmid partition inEscherichia coli. Annu Rev Biochem 61:283–306
Hiraga S (1993) Chromosome partition inEscherichia coli. Curr Opin Genet Dev 5:789–801
Hiraga S, Niki H, Ogura T, Ichinose C, Mori H, Ezaki B, Jaffé A (1989) Chromosome partitioning inEscherichia coli: novel mutants producing anucleate cells. J Bacteriol 171:1496–1505
Kleinschmidt JA, Dingwall C, Maier G, Franke WW (1986) Molecular characterization of a karyophilic, histone-binding protein: cDNA cloning, amino acid sequence and expression of nuclear protein N1/N2 ofXenopus laevis. EMBO J 5:3547–3552
Kouzarides T, Ziff E (1988) The role of the leucine zipper in the fos-jun interaction. Nature 336:646–651
Landschulz WH, Johnson PF, McKnight SL (1988) The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science 240:1759–1764
Michalak M, Milner RE, Burns K, Opas M (1992) Calreticulin. Biochem J 285:681–692
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Muro Y, Masumoto H, Yoda K, Nozaki N, Ohashi M, Okazaki T (1992) Centromere protein B assembles human centromeric α-satellite DNA at the 17-bp sequence CENP-B box. J Cell Biol 116:585–596
Nakabeppu Y, Ryder K, Nathans D (1988) DNA binding activities of three murine Jun proteins: stimulation by Fos. Cell 55:907–915
Niki H, Jaffé A, Imamura R, Ogura T, Hiraga S (1991) The new genemukB codes for a 177 kd protein with coiled-coil domains involved in chromosome partitioning ofE. coli. EMBO J 10:183–193
Niki H, Imamura R, Kitaoka M, Yamanaka K, Ogura T, Hiraga S (1992)E. coli MukB protein involved in chromosome partition forms a homodimer with a rod-and hinge structure having DNA binding and ATP/GTP binding activities. EMBO J 11:5101–5109
Ogura T, Hiraga S (1983) Mini-F plasmid genes that couple host cell division to plasmid proliferation. Proc Natl Acad Sci USA 80:4784–4788
Rauscher FJ III, Voulalas PJ, Franza BR Jr, Curran T (1988) Fos and Jun bind cooperatively to the AP-1 site: reconstitution in vitro. Genes Dev 2:1687–1699
Rudd KE (1992) Alignment ofE. coli DNA sequences to a revised, integrated genomic restriction map. In: Miller JH (ed) A short course in bacterial genetics: a laboratory manual and handbook forEscherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, pp 2.3–2.43
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning 2nd edition: a laboratory manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Skarstad K, Boye E (1993) Degradation of individual chromosomes inrecA mutants ofEscherichia coli. J Bacteriol 175:5505–5509
Skarstad K, Steen HB, Boye E (1985)Escherichia coli DNA distributions measured by flow cytometry and compared with theoretical computer simulations. J Bacteriol 163:661–668
Stahl FW, Kobayashi I, Thaler D, Stahl MM (1986) Direction of travel of RecBC recombinase through bacteriophage lambda DNA. Genetics 113:215–227
Yamanaka K, Mitani T, Feng J, Ogura T, Niki H, Hiraga S (1994) Two mutant alleles ofmukB, a gene essential for chromosome partition inEscherichia coli. FEMS Microbiol Lett 123:27–32
Yamanaka K, Ogura T, Niki H, Hiraga S (1992) Identification and characterization ofsmbA gene, a suppressor of themukB null mutant inEscherichia coli. J Bacteriol 174:7517–7526
Yamanaka K, Ogura T, Niki H, Hiraga S (1995) Characterization of thesmtA gene encoding an S-adenosylmethionine-dependent methyltransferase ofEscherichia coli. FEMS Microbiol Lett 133:59–63
Wada M, Kano Y, Ogawa T, Okazaki T, Imamoto F (1988) Construction and characterization of the deletion mutant ofhupA andhupB genes inEscherichia coli. J Mol Biol 204:581–591
Author information
Authors and Affiliations
Additional information
Communicated by M. Sekiguchi
Rights and permissions
About this article
Cite this article
Yamanaka, K., Ogura, T., Niki, H. et al. Identification of two new genes,mukE andmukF, involved in chromosome partitioning inEscherichia coli . Molec. Gen. Genet. 250, 241–251 (1996). https://doi.org/10.1007/BF02174381
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02174381