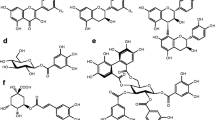
Abstract
Nine flavonoids, namely, kaempferol, kaempferol 7-rharanoside, kaempferol 3-rhamnoside, kaempferol 3-glucoside, kaempferol 3-glucoside, 7-rhamnoside, quercetin 3-glucoside, quercetin 3,7-diglucoside, isorhamnetin 3-glucoside, and isorhamnetin 3,7-diglucoside, have been identified in the body and wings of the chalkhill blue butterflyLysandra coridon Poda. Flavonoids have also been found in 15 of a further 17 lycaenid species examined. Analysis of the two-dimensional paper chromatographic flavonoid patterns and aglycone results has shown that the flavonoid content ofL. coridon and the other lycaenids is dependent on the flavonoid content of the larval diet. Differences in the flavonoid patterns ofL. coridon and its leguminous larval food plantsHippocrepis comosa, Anthyllis vulneraria, andLotus corniculatus, indicate that the ingested flavonoids are metabolized byL. coridon or its gut flora before sequestration. Despite the presence of fiavones, glycoflavones, and isoflavones in the larval food plant species, only flavonols are sequestered by the lycaenid species examined. The relationship between lycaenid butterflies and their larval food plants, and the possible role(s) of flavonoids in lycaenids has been discussed. Interactions between ants, plants, flavonoids, and myrmecophilous lycaenids have also been considered.
Similar content being viewed by others
References
Brower, L.P. 1985. Chemical defence in butterflies. Biology of butterflies.Symp. R. Entomol Soc. London. 11:109–143.
Brower, L.P., Sieber, J.N., Nelson, C.J., Lynch, S.P., andTuskes, P.M. 1982. Plant-determined variation in the cardenolide content, thin-layer Chromatographic profiles, and emetic potency of monarch butterflies,Danaus plexippus reared on the milkweedAscelepias eriocarpa in California.J. Chem. Ecol. 8:579–633.
Cottrell, C.B. 1983. Aphytophagy in butterflies: Its relationship to myrmecophily.Zool. J. Linn. Soc. 79:1–57.
Downey, J.C. 1962. Myrmecophily inPlebejus (Icaricia) icariodes (Lepidoptera: Lyeaenidae).Entomol. News 73:57–66.
Edgar, J.A. andCulvenor, C.C.J. 1974. Pyrrolizidine ester alkaloid in danaid butterflies.Nature 250:614–616.
Edgar, J.A., Culvenor, C.C.J., andPliske, T.E. 1974. Coevolution of danaid butterflies with their host plants.Nature 250:646–648.
Ehrlich, P.R., andRaven, P.H. 1964. Butterflies and plants: A study in coevolution.Evolution 18:586–608.
Eliot, J.N. 1973. The higher classification of the Lyeaenidae (Lepidoptera): a tentative arrangement.Bull. Bur. Mus. (Nat. Hist.) Entomol. 28:375–505.
Feltwell, J.S., andValadon, L.R.G. 1970. Plant pigments identified in the common blue butterfly.Nature 255:969.
Ford, E.B. 1941. Studies on the chemistry of pigments in the lepidoptera, with reference to their bearing on systematics. 1. The anthoxanthins.Proc. R. Entomol. Soc. London (A) 16:65–90.
Ford, E.B. 1944. Studies on the chemistry of pigments in the lepidoptera, with reference to their bearing on systematics. 4. The classification of the Papilionidae.Trans. R. Entomol. Soc. London (A) 19:92–106.
Ford, E.B. 1945. Butterflies. Collins, London.
Fujimoto, N., Hayashiya, K., andJono, S. 1959. Studies on the pigments of cocoon. (V) On the pigments of cocoon ofTheophila mandarina Moore.J. Sericult. Sci. Jpn. 28:84–87.
Gonnet, J.F. 1975. Flavonol glycosides ofAnthyllis vulneraria leaves.Phytochemistry 14:823–828.
Gonnet, J.F., andJay, M. 1972. Les aglycones flavoniquesd'Anthyllis vulnéraria.Phytochemistry 11:2313–2316.
Goodwin, T.W. 1950. Carotenoids and reproduction.Biol. Rev. 25:391–413.
Harashima, K., Ohno, T., Sawachika, T., Hidaka, T., andOhnishi, E. 1972, Carotenoids in orange pupae of the swallowtail,Papilio xuthus.Insect Biochem. 2:29–48.
Harashima, K., Nakahara, J., andKato, G. 1976. Papilioerythrinone: A new ketocarotenoid in integuments of orange pupae of a swallowtailPapilio xuthus, and carapaces of a crab,Paralithodes brevipes.Biol. Chem. 40:711–717.
Harborne, J.B. 1967. Comparative Biochemistry of the Flavonoids. Academic Press, London.
Harborne, J.B. 1973. Phytochemical Methods. Chapman and Hall, London.
Harborne, J.B., andMabry, T.J. 1982. The Flavonoids: Advances in Research. Chapman and Hall, London.
Havsteen, B. 1983. Flavonoids, a class of natural products of high pharmacological potency.Biochem. Pharmacol. 32:1141–1148.
Hayashiya, K., Sugimoto, S., andFujimoto, N. 1959. Studies on the pigments of cocoon. III. The qualitative test of the pigments of green cocoon.J. Sericult. Sci. Jpn. 28:27–32.
Higgins, L.G., andRiley, N.D. 1975. A Field Guide to the Butterflies of Britain and Europe. Collins, London.
Hinton, H.E. 1951. Myrmecophilous Lycaenidae and other Lepidoptera, a summary.Trans. South London Entomol. Nat. Hist. Soc. 1949–1950:111–175.
Jay, M., Lebreton, P., andLetoublon, R., 1971. Recherches chimotaxonomiques sur les plantes vasculaires. XXII. Apports récents de la biochimie a la résolution de quelques problèmes systematiques posés par les Legumineuses.Boissiera 19:219–257.
Kayser, H. 1982. Carotenoids in insects, pp 195–210,in G. Britton and T.W. Goodwin (eds.). Carotenoid Chemistry and Biochemistry. Pergamon Press, Oxford.
Lane, C. 1957. Preliminary note on some insects eaten and rejected by a tame shama (Kittacincla malabarica Gm.), with the suggestion that in certain species of butterflies and moths females are less palatable than males.Entomol. Mon. Mag. 93:172–179.
Malicky, H. 1969. Versuch einer Analyse der Okologischen Beziehungen zwischen Lycaeniden (Lepidoptere) und Formiciden (Hymenoptera).Tidjschr. Entomol. 112:213–298.
Markham, K.R. 1982. Techniques of Flavonoid Identification. Academic Press, London.
Mattson, W.J., Jr. 1980. Herbivory in relation to plant nitrogen content.Annu. Rev. Ecol. Syst. 11:119–161.
Morris, S.J., andThomson, R.H. 1963. The flavonoid pigments of the marbled white butterfly (Melanargia galathea Seltz).J. Insect Physiol. 9:391–399.
Morris, S.J., andThomson, R.H. 1964. The flavonoid pigments of the small heath butterfly,Coenonympha pamphilus L.J. Insect Physiol. 10:377–383.
Pierce, N.E. 1985. Lycaenid butterflies and ants: Selection for nitrogen-fixing and other protein-rich food plants.Am. Nat. 125:888–895.
Pierce, N.E., andElgar, M.A. 1985. The influence of ants on host-plant selection byJalmenus evagoras, a myrmecophilous lycaenid butterfly.Behav. Ecol. Sociobiol. 16:209–222.
Pierce, N.E., andMead, P.S. 1981. Parasitoids are selective agents in the symbiosis between lycaenid butterfly larvae and ants.Science 211:1185–1187.
Pocock, R.I. 1911. On the palatability of some British insects, with notes on the significance of mimetic resemblances.Proc. Zool. Soc. London 11:809–868.
Reynaud, J., Jay, M., andRaynaud, J. 1982. Flavonoid glycosides ofLotus corniculatus L. (Leguminosae).Phytochemistry 21:2604–2605.
Roeske, C.N., Sieber, J.S., Brower, L.P., andMoffitt, C.M. 1976. Milkweed cardenolides and their processing by monarch butterflies (Danaus plexippus L.)Recent Adv. Phytochem. 10:93–167.
Rothschild, M., andAplin, R.T. 1972. Poisonous alkaloids in the body tissues of the garden tiger moth (Arctia caja) and the cinnabar moth (Tyria (= Callimorpha) jacobaeae L.) (Lepidoptera), pp. 579–595,in A. de Vries and E. Kochra (eds.). Toxins of Plant and Animal Origin. Gordon and Breach, London.
Shapiro, A., andMasuda, K. 1981. New diet turns butterfly into pest.New Sci. 90:160.
Thomson, D.L. 1926. The pigments of butterflies' wings. 1.Melanargia galathea.Biochem. J. 20:73–75.
Wilson, A. 1985a. Flavonoid pigments of butterflies in the genusMelanargia.Phytochemistry 24:1685–1991.
Wilson, A. 1985b. Flavonoid pigments in marbled white butterfly (Melanargia galathea) are dependent on flavonoid content of larval diet.J. Chem. Ecol. 11:1161–1179.
Wilson, A. 1986a. Flavonoid pigments and wing color inMelanargia galathea.J. Chem. Ecol. 12:49–68.
Wilson, A. 1986b. Flavonoid pigments in swallowtail butterflies.Phytochemistry. 25:1309–1313.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wilson, A. Flavonoid pigments in chalkhill blue (Lysandra coridon Poda) and other lycaenid butterflies. J Chem Ecol 13, 473–493 (1987). https://doi.org/10.1007/BF01880094
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01880094