Summary
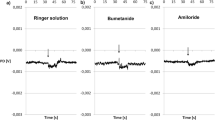
Experiments were carried out in the isolated short-circuited skin of the toadBufo marinus ictericus.42K influx and efflux experiments were carried out with skins bathed on both sides by NaCl-Ringer's solution. Those fluxes showed very similar kinetics of equilibration with time and the results could be fitted by equations of a model of two intraepithelial compartments and the bathing solutions. In the steady state K influx is 3.99 ±0.36 nmol cm−2 hr−1 (n=7) and efflux 3.62±0.38 nmol cm− hr−1 (n=7) and are not statistically different, indicating that no net K flux is present across the epithelium. Different kinds of perturbations affecting the rates of42K discharge into the bathing solutions were studied. Immediately after addition of amiloride (10−4 m) to the outer solution, a sharp decline is observed in the rate of42K discharge into the bathing solution,J K21 , which falls from 3.62±0.38 nmol cm−2 hr−1 to 2.02±0.04 nmol cm−2 hr−1 (n=7) 2 min after addition of the drug, followed by a partial recuperation with time. A complete Na by K substitution in the outer bathing solution induces a prompt and marked decline inJ K21 which is similar to that induced by amiloride. Increase in the outer bathing solution Na concentration from zero Na concentration induces a nonlinear increase inJ K21 and a linear relationship was observed betweenJ K21 and short-circuit current in the range of 0 to 115mm external Na concentration. The decline inJ K21 induced by amiloride or by lowering external Na concentration was interpreted as being caused by electrical hyperpolarization of the external barrier of the epithelium induced by these procedures. Depolarization of the epithelial barriers by inner Na by K substitution in the short-circuited state (when the potential barriers are equal) drastically interfere with the rate of42K discharge from the epithelium into the bathing solutions. Thus, transient increases are observed both in the rate of42K discharge to the outer and to the inner bathing solutions upon depolarization of the barriers. These results indicate that at least the most important component of transepithelial K unidirectional fluxes goes through a transcellular route with a negligible paracellular component. Addition of ouabain (10−3 m) to the inner bathing solution induces a transient rise in the rate of42K discharge to the outer bathing solution with a peak on the order of 200% of the stationary value previous to the action of the inhibitor, followed by a return to new stationary values not statistically different from those observed previously to the effect of ouabain. The behavior ofJ K21 upon the effect of ouabain, as suggested by comparison with predictions from computer simulation, strongly supports the notion of a rheogenic Na pump in the inner barrier of the epithelium against the notion of a nonrheogenic 1∶1 Na−K pump.
Similar content being viewed by others
References
Benos, D.J., Mandel, L.J. 1978. Amiloride is a non-competitive inhibitor of Na-transport in isolated bull frog skin.Biophys. J. 21:169a
Bentley, P.J. 1968. Amiloride: A potent inhibitor of sodium transport across the toad bladder.J. Physiol. (London) 195:317
Biber, T.U.L., Mullen, T.L. 1977. Effect of inhibitors on transepithelial efflux of Na and nonelectrolytes in frog skin.Am. J. Physiol. 232:C67
Cereijido, M., Curran, P.F. 1965. Intracellular electrical potentials in frog skin.J. Gen. Physiol. 48:543
Cereijido, M., Herrera, F.C., Flanigan, W.J., Curran, P.F. 1964. The influence of Na concentration on Na transport across frog skin.J. Gen. Physiol. 47:879
Cirne, B., Malnic, G. 1972. Action of mineralocorticoid and sex steroids on sodium transport in toad skin.Biochim. Biophys. Acta 274:171
Crabbé, J., Ehrlich, N., Scarlata, J. 1968. Amiloride and the mode of action of aldosterone on sodium transport across toad bladder and skin.Pfluegers Arch. 304:284
Curran, P.F., Cereijido, M. 1965. K fluxes in frog skin.J. Gen. Physiol. 48:1011
Danisi, G., Lacaz-Vieira, F. 1974. Nonequilibrium thermodynamic analysis of the coupling between active sodium transport and oxygen consumption.J. Gen. Physiol. 64:372
Dixon, W.J., Massey, F.J. 1969. Introduction to statistical analysis. McGraw-Hill Kogakusha, Tokyo
Dörge, A., Nagel, W. 1970. Effect of amiloride on sodium transport in frog skin. II. Sodium transport pool and unidirectional fluxes.Pfluegers Arch. 321:91
Dörge, A., Rick, R., Thurau, K. 1976. Characterization of the transport pool for sodium in frog skin by X-ray microanalysis.J. Physiol. (London) 263:202P
Farquhar, M.G., Palade, G.E. 1965. Cell junctions in amphibian skin.J. Cell. Biol. 26:263
Farquhar, M.G., Palade, G.E. 1966. Adenosine triphosphatase localization in amphibian epidermis.J. Cell. Biol. 30:359
Finn, A.L. 1976. Changing concepts of transepithelial sodium transport.Physiol. Rev. 56:453
Fuchs, W., Hviid-Larsen, E., Lindemann, B. 1977. Current-voltage curve of sodium channels and concentration dependence of sodium permeability in frog skin.J. Physiol. (London) 267:137
Gatzy, J.T., Clarkson, T.W. 1965. The effect of mucosal and serosal solution cations on bioelectric properties of the isolated toad bladder.J. Gen. Physiol. 48:647
Goldman, D.E. 1943. Potential, impedance and rectification in membranes.J. Gen. Physiol. 27:37
Helman, S.I., Fisher, R.S. 1977. Microelectrode studies of the active Na transport pathway of frog skin.J. Gen. Physiol. 69:571
Helman, S.I., Miller, D.A. 1971. In vitro techniques for avoiding edge damage in studies of frog skin.Science 173:146
Helman, S.I., Miller, D.A. 1973. Edge damage effect on electrical measurements of frog skin.Am. J. Physiol. 225:972
Helman, S.I., Miller, D.A. 1974. Edge damage effect on measurements of urea and sodium flux in frog skin.Am. J. Physiol. 226:1198
Hodgkin, A.L., Katz, B. 1949. The effect of sodium ions on electrical activity of the giant axon of the squid.J. Physiol. (London) 108:37
Huf, E.G., Wills, J. 1953. The relationship of sodium uptake, potassium rejection, and skin potential in isolated frog skin.J. Gen. Physiol. 36:473
Kirschner, L.B. 1955. On the mechanism of active sodium transport across the frog skin.J. Cell. Comp. Physiol. 45:61
Koefoed-Johnsen, V. 1957. The effect of g-strophanthin (ouabain) on the active transport of sodium through the isolated frog skin.Acta Physiol. Scand. (Suppl.) 42:145
Koefoed-Johnsen, V., Ussing, H.H. 1958. The nature of the skin potential.Acta Physiol. Scand. 42:298
Kyte, J. 1972. The titration of the cardiac glycoside binding sites of the (Na+−K+) adenosine triphosphatase.J. Biol. Chem. 247:7634
Larsen, E.H. 1972. Effect of amiloride, cyanide and ouabain on the active transport pathway in toad skin.In: Alfred Benzon Symposium. V. Transport Mechanisms in Epithelia. H.H. Ussing and N.A. Thorn, editors. p. 131. Academic, New York
Lindley, B.D., Hoshiko, T. 1964. The effects of alkali metal cations and common anions on the frog skin potential.J. Gen. Physiol. 47:749
Macknight, A.D.C. 1977. Epithelial transport of potassium.Kidney Int. 11:391
MacRobbie, E.A.C., Ussing, H.H. 1961. Osmotic behaviour of the epithelial cells of frog skin.Acta Physiol. Scand. 53:348
Mandel, L.J., Curran, P.F. 1972. Response of the frog skin to steady-state voltage clamping. I. The shunt pathway.J. Gen. Physiol. 59:503
Mandel, L.J., Curran, P.F. 1973. Response of the frog skin to steady-state voltage clamping. II. The active pathway.J. Gen. Physiol. 62:1
Mills, J.W., Ernst, S.A., DiBona, D.R. 1977. Localization of Na+-pump sites in frog skin.J. Cell. Biol. 73:88
Moyer, B.J. 1962. A survey of Cerenkov counter technique.In: “Nuclear instruments and their uses” A.H. Snell, editor. Vol. 1. Wiley and Sons, New York
Nagel, W. 1976. The intracelular electrical potential profile of the frog skin epithelium.Pfluegers Arch. 365:135
Nielsen, R. 1971. Effect of amphotericin B on the frog skin in vitro. Evidence for outward active potassium transport across the epithelium.Acta Physiol. Scand. 83:106
Pipes, L.A., Harvill, L.R. 1970. Applied mathematics for engineers and physicists. McGraw-Hill Kogakusha, Tokyo
Procopio, J., Lacaz-Vieira, F. 1977. Ionic exchanges in isolated and open-circuited toad skin.J. Membrane Biol. 35:219
Rawlins, F., Mateu, L., Fragachan, F., Whittembury, G. 1970. Isolated toad skin epithelium: Transport characteristics.Pfluegers Arch. 316:64
Rick, R., Dörge, A., Arnim, E. von, Thurau, K. 1978. Electron microprobe analysis of frog skin epithelium: Evidence for a syncytial sodium transport compartment.J. Membrane Biol. 39:313
Salako, L.A., Smith, A.J. 1969. Inhibition of active sodium transport in isolated frog skin by the diuretic, amiloride: Site of action.J. Physiol. (London) 206:37P
Schultz, S.G. 1972. Electrical potential differences and electromotive forces in epithelial tissues.J. Gen. Physiol. 59:794
Schultz, S.G. 1978. Is a coupled Na−K exchange “pump” involved in active transepithelial Na transport? A status report.In: Membrane Transport Processes. J.F. Hoffman, editor. Vol. 1. Raven Press, New York
Ussing, H.H., Windhager, E.E. 1964. Nature of shunt path and active sodium transport path through frog skin epithelium.Acta Physiol. Scand. 61:484
Varanda, W.A., Lacaz-Vieira, F. 1978. Transients in toad skin: Short-circuit current and ionic fluxes related to inner sodium substitution by monovalent cations.J. Membrane Biol. 39:369
Voûte, C.L., Ussing, H.H. 1968. Some morphological aspects of active sodium transport.J. Cell. Biol. 36:625
Walser, M. 1970. Role of edge damage in sodium permeability of toad bladder and a means of avoiding it.Am. J. Physiol. 219:225
Whittembury, G. 1964. Electrical potential profile of the toad skin epithelium.J. Gen. Physiol. 47:795
Zylber, E.A., Rotunno, C.A., Cereijido, M. 1973. Ion and water balance in isolated epithelial cells of the abdominal skin of the frogLeptodactylus ocellatus.J. Membrane Biol. 13:199
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Varanda, W.A., Lacaz-Vieira, F. Transient potassium fluxes in toad skin. J. Membrain Biol. 49, 199–233 (1979). https://doi.org/10.1007/BF01871119
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01871119