Summary
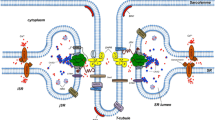
It has been previously recognized that a number of protocols may cause breakage of the triad junction and separation of the constituent organelles of skeletal muscle. We now describe a fraction of triad junctions which is refractory to the known protocols for disruption. Triads were passed through a French press and the dissociated organelles were separated on a sucrose density gradient, which was assayed for PN200-110, ouabain and ryanodine binding. Ryanodine binding showed a single peak at the density of heavy terminal cisternae. On the other hand, the PN200-110 and ouabain, which are external membrane ligands, bound in two peaks: one at the free transverse tubule region and the other at the light terminal cisternae. Similarly, a two peak pattern of PN200-110 and ouabain binding was observed when triad junctions were broken by the Ca2+-dependent protease, calpain, which selectively hydrolyzes the junctional foot protein. The light terminal cisternae vesicles were subjected to three different procedures of junctional breakage: French press, hypertonic salt treatment, and protease digestion using calpain or trypsin. The treated membranes were then centrifuged on density gradients. Only extensive trypsin digestion caused a partial shift of ouabain activity into the free transverse tubule region. These observations suggest that the triads are a composite mixture of breakage susceptible, “weak,” and breakage resistant, “strong,” triads. Scatchard analysis of PN200-110 suggests that the transverse tubules of strong triads contain a relatively high number of dihydropyridine receptors compared to those of weak triads. Thin section electron microscopic images of the strong triads comparable to those of intact muscle are presented.
Similar content being viewed by others
References
Block, B.A., Imagawa, T., Campbell, K.P., Franzini-Armstrong, C. 1988. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle.J. Cell Biol. 107:2587–2600
Borsotto, M., Barhanin, J., Fosset, M., Lazdunski, M. 1985. The 1,4-dihydropyridine receptor associated with the skeletal muscle voltage-sensitive Ca2+ channel.J. Biol. Chem. 260:14255–14263
Bradford, M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principles of protein-dye binding.Anal. Biochem. 72:248–249
Brandt, N.R., Caswell, A.H., Brunschwig, J.-P. 1980. ATP-energized Ca2+ pump in isolated transverse tubules of skeletal muscle.J. Biol. Chem. 255:6250–6258
Brandt, N.R., Caswell, A.H., Wen, S.-R., Talvenheimo, J.A. 1990. Molecular interactions of the junctional foot protein and dihydropyridine receptor in skeletal muscle triads.J. Membrane Biol. 113:237–251
Brunschwig, J.-P., Brandt, N.R., Caswell, A.H., Lukeman, D.S. 1982. Ultrastructural observations of isolated intact and fragmented junctions of skeletal muscle by use of tannic acid mordanting.J. Cell Biol. 93:533–542
Cadwell, J.S., Caswell, A.H. 1982. Identification of a constituent of the junctional feet linking terminal cisternae to transverse tubules in skeletal muscle.J. Cell Biol. 93:543–550
Campbell, K.P., Knudson, C.M., Imagawa, T., Leung, A.T., Sutko, J.L., Kahl, S.D., Raab, C.R., Madson, L. 1987. Identification and characterization of the high affinity [3H]ryanodine receptor of the junctional sarcoplasmic reticulum Ca2+ release channel.J. Biol. Chem. 262:6460–6463
Caswell, A.H., Brandt, N.R. 1981a. Correlation of Ca2+ release from terminal cisternae with integrity of triad junction.In: The Mechanism of Gated Calcium Transport Across Biological Membranes. S.T. Ohnishi and M. Endo, editors. pp. 219–226. Academic. New York
Caswell, A.H., Brandt, N.R. 1981b. Ion-induced release of calcium from isolated sarcoplasmic reticulum.J. Membrane Biol. 58:21–33
Caswell, A.H., Lau, Y.H., Brunschwig, J.-P. 1976. Ouabain-binding vesicles from skeletal muscle.Arch. Biochem. Biophys. 176:417–430
Caswell, A.H., Lau, Y.H., Garcia, M., Brunschwig, J.-P. 1979. Recognition and junction formation by isolated transverse tubules and terminal cisternae of skeletal muscle.J. Biol. Chem. 254:202–208
Eisenberg, B.R., Eisenberg, R.S. 1982. The T-SR junction in contracting single skeletal muscle fibres.J. Gen. Physiol. 79:1–19
Eisenberg, B.R., Gilai, A. 1979. Structural changes in single muscle fibers after stimulation at a low frequency.J. Gen. Physiol. 74:1–16
Ferguson, D.G., Schwartz, H.W., Franzini-Armstrong, C. 1984. Subunit structure of junctional feet in triads of skeletal muscle: A freeze-drying, rotatory-shadowing study.J. Cell. Biol. 99:1735–1742
Franzini-Armstrong, C. 1970. Studies of the triad. I. Structure of the junction in frog twitch fibers.J. Cell Biol. 47:488–499
Franzini-Armstrong, C., Nunzi, G. 1983. Junctional feet and particles in triads of a fast twitch muscle fibre.J. Muscle Res. Cell Motil. 4:233–252
Hymel, L.M., Inui, S., Fleisher, S., Schindler, H. 1988. Purified ryanodine receptor of skeletal muscle sarcoplasmic reticulum forms Ca2+-activated oligomeric Ca2+ channels in planar bilayers.Proc. Natl. Acad. Sci. USA 85:441–445
Imagawa, T., Smith, J.S., Coronado, R., Campbell, K.P. 1987. Purified ryanodine receptor from skeletal muscle sarcoplasmic reticulum is the Ca2+-permeable pore of the calcium release channel.J. Biol. Chem. 262:16636–16643
Inui, M., Saito, A., Fleisher, S. 1987. Purification of the ryanodine receptor and identity with feet structures of junctional terminal cisternae of sarcoplasmic reticulum from fast skeletal muscle.J. Biol. Chem. 262:1740–1747
Kawamoto, R.M., Brunschwig, J.-P., Caswell, A.H. 1988. Localization by immunoelectron microscopy of spanning protein of triad junction in terminal cisternae/triad vesicles.J. Muscle Res. Cell Motil. 9:334–343
Kawamoto, R.M., Brunschwig, J.-P., Kim, K.C., Caswell, A.H. 1986. Isolation, characterization, and localization of the spanning protein from skeletal muscle triads.J. Cell. Biol. 103:1405–1414
Kelly, D.E. 1969. The fine structure of skeletal muscle triad junctions.J. Ultrastruct. Res. 29:37–49
Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4.Nature (London) 227:680–685
Lai, F.A., Erickson, H., Block, B.A., Meissner, G. 1987. Evidence for a junctional feet-ryanodine receptor complex from sarcoplasmic reticulum.Biochem. Biophys. Res. Commun. 143:704–709
Lai, F.A., Erickson, H.P., Rousseau, E., Liu, Q.-Y., Meissner, G. 1988. Purification and reconstitution of the calcium release channel from skeletal muscle.Nature (London) 331:315–319
Murachi, T., Tanaka, K., Hatanaka, M., Murakami, T. 1981. Intracellular Ca2+-dependent protease (calpain) and its high-molecular-weight endogenous inhibitors (calpastatin).Adv. Enzyme Regul. 19:407–424
Rios, E., Brum, G. 1987. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle.Nature (London) 325:717–720
Saito, A., Steven, S., Chu, A., Fleisher, S. 1984. Preparation and morphology of sarcoplasmic reticulum terminal cisternase from rabbit skeletal muscle.J. Cell Biol. 99:875–885
Seiler, S., Wegener, A.D., Whang, D.D.,Hathway, D.R., Jones, L.R. 1984. High molecular weight proteins in cardiac and skeletal muscle junctional sarcoplasmic reticulum vesicles bind calmodulin, are phosphorylated, and are degraded by Ca2+-activated protease.J. Biol. Chem. 256:8550–8557
Somlyo, A.V. 1979. Bridging structures spanning the junctional gap at the triad of skeletal muscle.J. Cell Biol. 80:743–750
Tanabe, T., Beam, K.G., Powell, J.A., Numa, S. 1988. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA.Nature (London) 336:134–139
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kim, K.C., Caswell, A.H., Brunschwig, J.P. et al. Identification of a new subpopulation of triad junctions isolated from skeletal muscle; Morphological correlations with intact muscle. J. Membrain Biol. 113, 221–235 (1990). https://doi.org/10.1007/BF01870074
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01870074