Summary
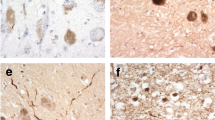
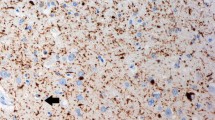
Alzheimer's disease (AD) and Parkinson's disease (PD) are the most common age-related degenerative disorders of the human brain. Both diseases involve multiple neuronal systems and are the consequences of cytoskeletal abnormalities which gradually develop in only a small number of neuronal types. In AD, susceptible neurons produce neurofibrillary tangles (NFTs) and neuropil threads (NTs), while in PD, they develop Lewy bodies (LBs) and Lewy neurites (LNs). The specific lesional pattern of both illnesses accrues slowly over time and remains remarkably consistent across cases.
In AD, six developmental stages can be distinguished on account of the predictable manner in which the neurofibrillary changes spread across the cerebral cortex. The pathologic process commences in the transentorhinal region (clinically silent stages I and II), then proceeds into adjoining cortical and subcortical components of the limbic system (stages III and IV — incipient AD), and eventually extends into association areas of the neocortex (stages V and VI — fully developed AD).
During the course of PD, important components of the limbic system undergo specific lesions as well. The predilection sites include the entorhinal region, the CA2-sector of the hippocampal formation, the limbic nuclei of the thalamus, anterior cingulate areas, agranular insular cortex (layer VI), and — within the amygdala — the accessory cortical nucleus, the ventromedial divisions both of the basal and accessory basal nuclei, and the central nucleus. The amygdala not only generates important projections to the prefrental association areas but also exerts influence upon all non-thalamic nuclei which in a non-specific manner project upon the cerebral cortex and upon the nuclei regulating endocrine and autonomie functions. All these amygdala-dependent structures themselves exhibit severe PD-specific lesions. In general, the extranigral destructions are in themselves not sufficient to produce overt intellectual deterioration. Similarly, AD-related pathology up to stage III may be asymptomatic as well. Fully developed PD with concurring incipient AD, however, is likely to cause impaired cognition. Presently available data support the view that the occurrence of additional lesions in the form of AD stage III (or more) destruction is the most common cause of intellectual decline in PD.
Similar content being viewed by others
References
Agid Y, Ruberg M, Javoy-Agid F, Hirsch E, Raisman-Vozari R, Vyas S, Faucheux B, Michel P, Kastner A, Blanchard V, Dier P, Villares J, Zhang P (1993) Are dopamin ergic neurons selectively vulnerable to Parkinson's disease? Adv Neurol 60: 148–164
Airaksinen MS, Paer A, Paljärvi L, Reinikanen K, Riekkinen P, Suomalainen R, Panula P (1991) Histamine neurons in human hypothalamus: anatomy in normal and Alzheimer diseased brains. Neuroscience 44: 465–481
Alheid GF, Heimer L, Switzer RC (1990) Basal ganglia. In: Paxinos G (ed) The human nervous system. Academic Press, New York, pp 483–582
Alexander GE, Crutcher MD, DeLong MR (1990) Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Progr Brain Res 85: 119–146
Amaral DG (1987) Memory: anatomical organization of candidate brain regions. In: Brookhart JM, Mountcastle VB (eds) Handbook of physiology: the nervous system, V. Higher function of the nervous system, 5th ed. Am Physiol Soc, Bethesda, pp 211–294
Amaral DG, Witter MP (1989) The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience 31: 571–591
Amaral DG, Insausti R (1990) Hippocampal formation. In: Paxinos G (ed) The human nervous system. Academic Press, San Diego, pp 711–755
Amaral DG, Price JL, Pitkänen A, Carmichael ST (1992) Anatomical organization of the primate amygdaloid complex. In: Aggleton JP (ed) The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. Wiley-Liss, New York, pp 1–66
Arendt T, Bigl V, Arendt A, Tennstedt A (1983) Loss of neurones in nucleus basalis of Meynert in Alzheimer's disease, paralysis agitans and Korsakoff s disease. Acta Neuropathol 61: 101–108
Armstrong E (1990) Limbic thalamus: anterior and mediodorsal nuclei. In: Paxinos G (ed) The human nervous system. Academic Press, San Diego, pp 469–482
Arnold SE, Hyman BT, Flory J, Damasio AR, van Hoesen GW (1991) The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cerebral Cortex 1: 103–116
Babinski R, Calabrese P, Durwen HF, Markowitsch HJ, Brechtelsbauer D, Heuser L, Gehlen W (1993) The possible contribution of the amygdala to memory. Behav Neurol 6: 167–170
Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, Wiche G, Scitelberger F, Grundke-Iqbal I, Wisniewski HM (1989) Accumulation of abnormally phosphorylated τ precedes the formation of neurofibrillary tangles in Alzheimer's disease. Brain Res 477: 90–99
Bancher C, Braak H, Fischer P, Jellinger KA (1993) Neuropathological staging of Alzheimer lesions and intellectual status in Alzheimer's and Parkinson's disease. Neurosci Lett 162: 179–182
Braak E, Braak H, Mandelkow EM (1994) A sequence of cytoskeleton changes related to the formation of neurofibrillary tangles and neuropil threads. Acta Neuropathol 87: 554–567
Braak H (1980) Architectonics of the human telencephalic cortex. Springer, Berlin Heidelberg New York, pp 1–147
Braak H, Braak E (1983) Neuronal types in the basolateral amygdaloid nuclei of man. Brain Res Bull 11: 349–365
Braak H, Braak E (1985) On areas of transition between entorhinal allocortex and temporal isocortex in the human brain. Normal morphology and lamina-specific pathology in Alzheimer's disease. Acta Neuropathol 68: 325–332
Braak H, Braak E (1986) Nuclear configuration and neuronal types of the nucleus niger in the brain of the human adult. Hum Neurobiol 5: 71–82
Braak H, Braak E (1989) Cortical and subcortical argyrophilic grains characterize a disease associated with adult onset dementia. Neuropathol Appl Neurobiol 15: 13–26
Braak H, Braak E (1990) Cognitive impairment in Parkinson's disease: amyloid plaques, neurofibrillary tangles and neuropil threads in the cerebral cortex. J Neural Transm [P-D Sect] 2: 45–57
Braak H, Braak E (1991a) Alzheimer's disease affects limbic nuclei of the thalamus. Acta Neuropathol 81: 261–268
Braak H, Braak E. (1991b) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82: 239–259
Braak H, Braak E (1992a) Anatomy of the human hypothalamus (chiasmatic and tuberal region). Progr Brain Res 93: 3–16
Braak H, Braak E (1992b) The human entorhinal cortex: normal morphology and lamina-specific pathology in various diseases. Neurosci Res 15: 6–31
Braak H, Braak E (1993a) Alzheimer neuropathology and limbic circuits. In: Vogt BA, Gabriel M (eds) Neurobiology of cingulate cortex and limbic thalamus. Birkhäuser, Boston, pp 606–626
Braak H, Braak E (1993b) Anatomy of the human basal ganglia. In: Szelenyi I (ed) Inhibitors of monoamine oxidase B. Birkhäuser, Basel, pp 3–23
Braak H, Braak E (1994) Pathology of Alzheimer's disease. In: Calne DB (ed) Neurodegenerative diseases. Saunders, Philadelphia, pp 585–613
Braak H, Braak E (1995) Staging of Alzheimer related neurofibrillary changes. Neurobiol Aging 16: 271–284
Braak H, Braak E, Bohl J (1992) Retrosplenial region involvement in Alzheimer's disease. Neurodegeneration 1: 53–57
Braak H, Duyckaerts C, Braak E, Piette F (1993) Neuropathological staging of Alzheimer-related changes correlates with psychometrically assessed intellectual status. In: Corian B, Iqbal K, Nicolini M, Winblad B, Wisniewski H, Zatta PF (eds) Alzheimer's disaese: advances in clinical and basic research. Wiley, Chichester, pp 131–137
Braak H, Braak E, Yilmazer D, de Vos RAI, Jansen ENH, Bohl J, Jellinger K (1994) Amygdala pathology in Parkinson's disease. Acta Neuropathol 88: 493–500
Braak H, Braak E, Yilmazer D, de Vos RAI, Jansen ENH, Bohl J, Jellinger K (1995) Nigral and extranigral lesions in Parkinson's disease. J Neural Transm [Suppl] 46: 15–31
Braak H, Braak E, Yilmazer D, Bohl J (1996) Functional anatomy of the human hippocampal formation. Review. J Child Neurol (in press)
Calne DB (1983) Current views on Parkinson's disease. Can J Neurol Sci 10: 11–15
Chan-Palay V, Asan E (1989) Alterations in catecholamine neurons of the locus coeruleus in senile dementia of the Alzheimer type and in Parkinson's disease with and without dementia and depression. J Comp Neurol 287: 373–392
Damasio AR, Damasio H (1991) Disorders of higher brain function. In: Rosenberg RN (ed) Comprehensive neurology. Raven Press, New York, pp 639–657
DeLacalle S, Lim C, Sobreviela T, Mufson EJ, Hersh LB, Saper CP (1994) Cholinergic innervation in the human hippocampal formation including the entorhinal cortex. J Comp Neurol 345: 321–344
De Olmos J (1990) Amygdala. In: Paxinos G (ed) The human nervous system. Academic Press, New York, pp 583–710
De Vos RAI, Jansen ENH, Stam FC, Ravid R, Swaab D (1995) “Lewy body disease”: clinico-pathological correlations in 18 consecutive cases of Parkinson's disease with and without dementia. Clin Neurol Neurosurg 97: 13–22
Dickson DW, Ruan D, Crystal H, Mark MH, Davies P, Kress Y, Yen SH (1991) Hippocampal degeneration differentiates diffuse Lewy body disease (DLBD) from Alzheimer's disease: light and electron microscopic immunocytochemistry of CA2-3 neuntes specific to DLBD. Neurology 41: 1402–1409
Fearnley J, Lees A (1994) Pathology of Parkinson's disease. In: Calne DB (ed) Neurodegenerative diseases. Saunders, Philadelphia, pp 545–554
Felleman DJ, van Essen DC (1991) Distributed hierarchical processing in the primate cerebral cortex. Cerebral Cortex 1: 1–47
Forno LS (1986) The Lewy body in Parkinson's disease. Adv Neurol 45: 35–43
Galloway PG, Grundke-Iqbal I, Iqbal K, Perry G (1988) Lewy bodies contain epitopes both shared and distinct from Alzheimer neurofibrillary tangles. J Neuropathol Exp Neurol 47: 654–663
German DC, White CL, Sparkman DR (1987) Alzheimer's disease: neurofibrillary tangles in nuclei that project to the cerebral cortex. Neuroscience 21: 305–312
Gibb WRG (1989) The pathology of parkinsonian disorders. In: Quinn NP, Jenner PG (eds) Disorders of movement — clinical, pharmacological and physiological aspects. Academic Press, London, pp 33–57
Gibb WRG, Lees AJ (1988) The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry 51: 745–752
Gibb WRG, Lees AJ (1989) The significance of the Lewy body in the diagnosis of idiopathic Parkinson's disease. Neuropathol Appl Neurobiol 15: 27–44
Gibb WRG, Lees AJ (1991) Anatomy, pigmentation, ventral and dorsal subpopulations of the substantia nigra, and differential cell death in Parkinson's disease. J Neurol Neurosurg Psychiatry 54: 388–396
Gibb WRG, Scott T, Lees AJ (1991) Neuronal inclusions of Parkinson's disease. Mov Disord 6: 2–11
Goedert M (1993) Tau protein and the neurofibrillary pathology of Alzheimer's disease. Trends Neurosci 16: 460–465
Goldman-Rakic PS (1987) Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In: Brookhart JM, Mountcastle VB (eds) Handbook of physiology: the nervous system, V. Higher functions of the nervous system, 5th ed. Am Physiol Soc, Bethesda, pp 373–407
Goldman-Rakic PS, Porrino LJ (1985) The primate mediodorsal (MD) nucleus and its projections to the frontal lobe. J Comp Neurol 242: 535–560
Hedreen JC, Struble RG, Whitehouse PJ, Price DL (1984) Topography of the magnocellular basal forebrain system in human brain. J Neuropathol Exp Neurol 31: 1–21
Heimer L, Switzer RC, van Hoesen GW (1982) Ventral striatum and ventral pallidum. Components of the motor system? Trends Neurosci 5: 83–87
Heimer L, de Olmos J, Alheid GF, Zaborszky L (1991) “Perestroika” in the basal forebrain: opening the border between neurology and psychiatry. Progr Brain Res 87: 109–165
Herzog AG, Kemper TL (1980) Amygdaloid changes in aging and dementia. Arch Neurol 7: 625–629
Hirsch EC, Graybiel AM, Agid YA (1988) Melanized dopaminergic neurons are differently susceptible to degeneration in Parkinson's disease. Nature 334: 345–348
Hyman BT, van Hoesen GW, Damasio AR, Barnes CL (1984) Alzheimer's disease: cellspecific pathology isolates the hippocampal formation. Science 225: 1168–1170
Hyman BT, van Hoesen GW, Damasio AR (1990) Memory-related neural systems in Alzheimer's disease: an anatomic study. Neurology 40: 1721–1730
Insausti R, Tunon T, Sobreviela T, Insausti AM, Gonzalo LM (1995) The human entorhinal cortex: a cytoarchitectonic analysis. J Comp Neurol 335: 171–198.
Iqbal K, Alonso AC, Gong CX, Khatoon S, Singh TJ, Grundke-Iqbal I (1994) Mechanism of neurofibrillary degeneration in Alzheimer's disease. Mol Neurobiol 9: 119–123
Jellinger K (1989) Pathology of Parkinson's disease. In: Calne DB (ed) Handbook of experimental pharmacology, vol 88. Drugs for the treatment of Parkinson's disease. Springer, Berlin Heidelberg New York Tokyo, pp 44–112
Jellinger K (1991) Pathology of Parkinson's disease. Changes other than the nigrostriatal pathway. Mol Chem Neuropathol 14: 153–197
Jellinger K (1994) Structural basis of dementia in Parkinson's disease. In: Korczyn AD (ed) Dementia in Parkinson's disease. Monduzzi, Bologna, pp 31–38
Jellinger K, Bancher C (1995) Structural basis of mental impairment in Parkinson's disease. Neuropsychiatrie 9: 9–14
Jellinger K, Braak H, Braak E, Fischer P (1991) Alzheimer lesions in the entorhinal region and isocortex in Parkinson's and Alzheimer's diseases. Ann NY Acad Sci 640: 203–209
Jones EG (1985) The thalamus. Plenum, New York
Kalus P, Braak H, Braak E, Bohl J (1989) The presubicular region in Alzheimer's disease: topography of amyloid deposits and neurofibrillary changes. Brain Res 494: 198–203
Kemper TL (1978) Senile dementia: a focal disease in the temporal lobe. In: Nandy E (ed) Senile dementia: a biomedical approach. Elsevier, Amsterdam, pp 105–113
Kromer-Vogt LJ, Hyman BT, van Hoesen GW, Damasio AR (1990) Pathologic alterations in the amygdala in Alzheimer's disease. Neuroscience 37: 377–385
Langston JW, Forno LS (1978) The hypothalamus in Parkinson's disease. Ann Neurol 3: 129–133
Lewy FH (1923) Die Lehre vom Tonus der Bewegung, zugleich systematische Untersuchungen zur Klinik, Physiologie, Pathologie und Pathogenese der Paralysis agitans. Springer, Berlin
Lowe J (1994) Lewy bodies. In: Calne DB (ed) Neurodegenerative diseases. Saunders, Philadelphia, pp 51–69
Manaye KF, McIntire DD, Mann DMA, German DC (1995) Locus coeruleus cell loss in the aging human brain: a non-random process. J Comp Neurol 358: 79–87
Mann DMA (1984) Dopamine neurones of the vertebrate brain: some aspects of anatomy and pathology. In: Winslow W, Markstein R (eds) The neurobiology of dopamine systems. Univers Press, Manchester, pp 87–103
Marinkovic SV, Milisavljevic MM, Vuckovic VD (1991) Microvascular anatomy of the uncus and the parahippocampal gyrus. Neurosurgery 29: 805–814
Markowitsch HJ (1982) Thalamic mediodorsal nucleus and memory: a critical evaluation of studies in animals and man. Neurosci Biobehav Rev 6: 351–380
Markowitsch HJ (1995) Anatomical basis of memory disorders. In: Gazzaniga MS (ed) The cognitive neurosciences. MIT Press, Cambridge, Mass, pp 765–779
Matzuk MM, Saper CB (1985) Preservation of hypothalamic dopaminergic neurons in Parkinson's disease. Ann Neurol 18: 552–555
Mesulam MM, Geula C (1988) Nucleus basalis (DH4) and cortical cholinergic innervation in the human brain: observations based on the distribution of acetylcholinestease and choline acetyltransferase. J Comp Neurol 275: 216–240
Mesulam MM, Hersh LB, Mash DC, Geula C (1992) Differential cholinergic innervation within functional subdivisions of the human cerebral cortex — a choline acetyltransferase study. J Comp Neurol 318: 316–328
Mishkin M (1982) A memory system in the monkey. Phil Trans R Soc London B 298: 85–95
Mizutani T, Kasahara M (1995) Degeneration of the intrahippocampal routes of the perforant and alvear pathways in senile dementia of Alzheimer type. Neurosci Lett 184: 141–144
Moossy J, Zubenko GS, Martinez AJ, Rao GR (1988) Bilateral symmetry of morphological lesions in Alzheimer's disease. Arch Neurol 45: 251–254
Nakano I, Hirano A (1984) Parkinson's disease: neuron loss in the nucleus basalis without concomitant Alzheimer's disease. Ann Neurol15: 415–418
Nauta HJW (1979) A proposed conceptual reorganization of the basal ganglia and telencephalon. Neuroscience 4: 1875–1881
Nauta WJH (1986) Circuitous connections linking cerebral cortex, limbic system, and corpus striatum. In: Doane BK, Livingston KE (eds) The limbic system. Raven Press, New York, pp 43–54
Ohm TG, Braak H (1988) The pigmented subpeduncular nucleus: a neuromelanin-containing nucleus in the human pontine tegmentum. Morphology and changes in Alzheimer's disease. Acta Neuropathol 77: 26–32
Ohm TG, Heilmann R, Braak H (1989) The human oral raphe system. Architectonics and neuronal types in pigment-Nissl preparations. Anat Embryol 180: 37–43
Pandya DN, Yeterian (1985) Architecture and connections of cortical association areas. In: Peters A, Jones EG (eds) Cerebral cortex, vol 4. Association and auditory cortices. Plenum Press, New York, pp 3–61
Pandya DN, Yeterian EH (1990) Prefrontal cortex in relation to other cortical areas in rhesus monkey: architecture and connections. Progr Brain Res 85: 63–94
Panula P, Airaksinen MS, Pirvola U, Kotilainen E (1990) A histamin-containing neuronal system in human brain. Neuroscience 34: 127–132
Paulus W, Jellinger K (1991) The neuropathologic basis of different clinical subgroups of Parkinson's disease. J Neuropathol Exp Neurol 50: 743–755
Pearson J, Halliday G, Sakamoto N, Michel JP (1990) Catecholaminergic neurons. In: Paxinos G (ed) The human nervous system. Academic Press, New York, pp 1023–1050
Price JL, Russchen FT, Amaral DG (1987) The amygdaloid complex. In: Björklund A, Hökfelt T, Swansen LW (eds) Handbook of chemical neuroanatomy, vol 5, part I. Integrated systems. Elsevier, Amsterdam, pp 279–388
Price JL, Davis PB, Morris JC, White DL (1991) The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer's disease. Neurobiol Aging 12: 295–312
Rosene DL, van Hoesen GW (1987) The hippocampal formation of the primate brain. A review of some comparative aspects of cytoarchitecture and connections. In: Jones EG, Peters A (eds) Cerebral cortex, vol 6. Further aspects of cortical functions including hippocampus. Plenum Press, New York, pp 345–456
Saper CB (1987a) Function of the locus coeruleus. Trends Neurosci 10: 343–344
Saper CB (1987b) Diffuse cortical projection systems: anatomical organization and role in cortical function. In: Plum F (ed) Handbook of physiology, vol 5. The nervous system. Am Physiol Soc, Bethesda, pp 169–210
Saper CB (1990a) Hypothalamus. In: Paxinos G (ed) The human nervous system. Academic Press, New York, pp 389–413
Saper CB (1990b) Cholinergic system. In: Paxinos G (ed) The human nervous system. Academic Press, New York, pp 1095–1113
Saper CB, Chelimsky TC (1984) A cytoarchitectonic and histochemical study of nucleus basalis and associated cell groups in the normal human brain. Neuroscience 13: 1023–1037
Saper CB, German DC (1987) Hypothalamic pathology in Alzheimer's disease. Neurosci Lett 74: 364–370
Saper CB, German DC, White CL (1985) Neuronal pathology in the nucleus basalis and associated cell groups in senile dementia of the Alzheimer's type: possible role in cell loss. Neurology 35: 1089–1095
Squire LR, Zola-Morgan S (1988) Memory: brain systems and behavior. Trends Neurosci 11: 170–175
Squire LR, Zola-Morgan S (1991) The medial temporal lobe memory system. Science 253: 1380–1386
Stephan H (1975) Allocortex. In: Bargmann W (ed) Handbuch der mikroskopischen Anatomie des Menschen, vol 4/9. Springer, Berlin Heidelberg New York
Swaab DF, Grundke-Iqbal I, Iqbal K, Kremer HPH, Ravid R, van de Nes JAP (1992) Tau and ubiquitin in the human hypothalamus in aging and Alzheimer's disease. Brain Res 590: 239–249
Törk I, Hornung JP (1990) Raphe nuclei and the serotonergic system. In: Paxinos G (ed) The human nervous system. Academic Press, New York, pp 1001–1022
Tourtellotte WG, van Hoesen GW, Hyman BT, Tikoo RK, Damasio AR (1989) Alz-50 immunoreactivity in the thalamic reticular nucleus in Alzheimer's disease. Brain Res 515: 227–234
Unger JW, McNeill TH, Lapham LL, Hamill RW (1988) Neuropeptides and neuropathology in the amygdala in Alzheimer's disease: relationship between somatostatin, neuropeptide Y and subregional distribution of neuritic plaques. Brain Res 452: 293–302
van Domburg PHMF, ten Donkelaar HJ (1991) The human substantia nigra and ventral tegmental area. In: Beck F, Hild W, Kriz W, Pauly JE, Sano Y, Schiebler TH (eds) Advances in anatomy, embryology and cell biology, vol 121. Springer, Berlin Heidelberg New York Tokyo
van Dongen PAM (1981) The human locus coeruleus in neurology and psychiatry. Progr Neurobiol 17: 97–139
van Hoesen GW, Hyman BT (1990) Hippocampal formation: anatomy and the patterns of pathology in Alzheimer's disease. Progr Brain Res 83: 445–457
van Hoesen GW, Hyman BT, Damasio AR (1991) Entorhinal cortex pathology in Alzheimer's disease. Hippocampus 1: 1–8
van Hoesen GW, Solodkin A (1993) Some modular features of temporal cortex in humans as revealed by pathological changes in Alzheimer's disease. Cerebral Cortex 3: 465–475
Vogt BA (1985) Cingulate cortex. In: Peters A, Jones EG (eds) Cerebral cortex, vol 4. Association and auditory cortices. Plenum Press, New York, pp 89–149
Whitehouse PJ, Hedreen JC, White CL, Price DL (1983) Basal forebrain neurons in the dementia of Parkinson's disease. Ann Neurol 13: 243–248
Witter MP (1993) Organization of the entorhinal-hippocampal system: a review of current anatomical data. Hippocampus 3: 33–44
Xuereb JH, Perry EK, Candy JM, Bonham JR, Perry RH, Marshall E (1990) Parameters of cholinergic neurotransmission in the thalamus in Parkinson's disease and Alzheimer's disease. J Neurol Sci 99: 185–197
Zilles K (1990) Cortex. In: Paxinos G (ed) The human nervous system. Academic Press, New York, pp 757–802
Zola-Morgan S, Squire LR (1993) Neuroanatomy of memory. Ann Rev Neurosci 16: 547–563
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Braak, H., Braak, E., Yilmazer, D. et al. Pattern of brain destruction in Parkinson's and Alzheimer's diseases. J. Neural Transmission 103, 455–490 (1996). https://doi.org/10.1007/BF01276421
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01276421