Abstract
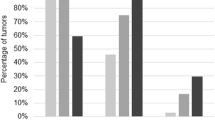
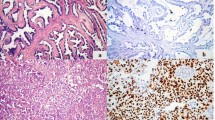
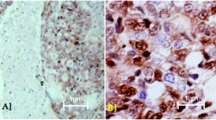
Objective: The objective of this study was to analyze the incidence of immunohistochemically detectable p53 protein accumulation in epithelial ovarian carcinomas and to correlate these data with the clinical outcome so as to clarify further the role of p53 mutations in prognosis with these patients.Methods: Tumor tissues from 179 patients with epithelial ovarian carcinoma were used for immuno-histochemical analysis with monoclonal antibody DO1 and BP 53-12-1 on formalin-fixed, paraffin-embedded tissue.Results: A total of 78 cases (44%) showed positive nuclear p53 staining. The p53-positive cases were found in all histological types of epithelial ovarian tumors. p53 staining was found in tumors of all stages with a higher percentage of positive cases in stage IV ovarian carcinomas (not significant). Poorly differentiated carcinomas showed a significantly higher percentage of p53 protein expression than did highly differentiated tumors (P=0.0002). Clinical follow-up of up to 14 years (median 25 months) showed a slightly but not significantly shortened disease-free and overall survival time for patients with p53-positive epithelial ovarian carcinomas.Conclusions: We conclude from our data that p53 expression in ovarian carcinoma is associated with poor differentiation but not with the disease being in an advanced stage. There was a tendency for shortened disease-free and overall survival for patients with p53-positive tumors.
Similar content being viewed by others
References
Banks L, Matlashewski G, Crawford L (1986) Isolation of human-p53-specific monoclonal antibodies and their use in the studies of human p53 expression. Eur J Biochem 159:529–534
Bartek J, Bartkova J, Vojtesek B, Staskova Z, Lukas J, Rejthar A, Kovarik J, Midgley CA, Gannon JV, Lane DP (1991) Aberrant expression of the p53 oncoprotein is a common feature of a wide spectrum of human malignancies. Oncogene 6:1699–1703
Berchuck A, Kohler MF, Marks JR, Wiseman R, Boyd J, Bast RC (1994) The p53 tumor suppressor gene frequently is altered in gynecologic cancers. Am J Obstet Gynecol 170:246–252
Casey G, Lo-Hsueh M, Lopez ME, Vogelstein B, Stanbridge EJ (1991) Growth suppression of human breast cancer cells by the introduction of a wild-type p53 gene. Oncogene 6:1791–1797
Day TG, Gallager HS, Rutledge FN (1975) Epithelial carcinoma of the ovary: Prognostic importance of histological grade. Natl Cancer Inst Monogr 42:15–18
Eccles DM, Brett L, Lessells A, Gruber L, Lane D, Steel CM, Leonard RCF (1992) Overexpression of the p53 protein and allele loss at 17p13 in ovarian carcinoma. Br J Cancer 65:40–44
El Deiry WS, Kern SE, Pietenpol JA, Kinzler KW, Vogelstein B (1992) Definition of a consensus binding site for p53. Nat Genet 1:45–49
Finlay CA, Hinds PW, Tan TH, Eliyahu D, Oren M, and Levine J (1988) Activating mutations for transformation by p53 produce a gene product that forms and hsc70-p53 complex with an altered half-life. Mol Cell Biol 8:531–539
Finlay CA, Hinds PW, Levine AJ (1989) The p53 proto-oncogene can act as a suppressor of transformation. Cell 57:1083–1093
Fisher CJ, Gillett CE, Vojtesek B, Barnes DM, Millis RR (1994) Problems with p53 immunohistochemical staining: the effect of fixation and variation in the methods of evaluation. Br J Cancer 69:26–31
Funk WD, Pak DT, Karas RH, Wright WE, Shay JW (1992) A transcriptionally active DNA-binding site for human p53. Mol Cell Biol 12:2866–2871
Halevy O, Michalovitz D, Oren M (1990) Different tumor-derived p53 mutants exhibit distinct biological activities. Science 250:113–116
Hall PA, McKee PH, Menage HduP, Dover R, Lane DP (1993) High levels of p53 protein in UV-irradiated normal human skin. Oncogene 8:203–207
Harris CC, Hollstein M (1993) Clinical implications of the p53 tumor-suppressor gene. N Engl J Med 329:1318–1327
Hartmann LC, Podratz KC, Keeney GL, Kamel NA, Edmonson JH, Grill JP, Su JQ, Katzmann JA, Roche PC (1994) Prognostic significance of p53 immunostaining in epithelial ovarian cancer. J Clin Oncol 12:64–69
Hinds PW, Weinberg RA (1994) Tumor suppressor genes. Curr Opinion Genet Dev 4:135–141
Hinds P, Finlay C, Levine A (1989) Mutation is required to activate the p53 gene for cooperation with theras oncogene and transformation. J Virol 63:739–746
Hollstein M, Sidransky D, Vogelstein B, Harris CC (1991) p53 Mutations in human cancers. Science 253:49–53
Kastan MB, Onyinye O, Sidransky D, Vogelstein B, Craig R (1991) Participation of p53 in the cellular response to DNA damage. Cancer Res 51:6304–6311
Kihana T, Tsuda H, Teshima S, Okada S, Matsuura S, Hirohashi S (1992) High incidence of p53 gene mutation in human ovarian cancer and its association with nuclear accumulation of p53 protein and tumor DNA aneuploidy. Jpn J Cancer Res 83:978–984
Kohler MF, Kerns BJM, Humphrey PA, Marks JR, Bast RC, Berchuck A (1993) Mutation and overexpression of p53 in early stage ovarian cancer. Obstet Gynecol 5:643–650
Kraiss S, Spiess S, Reihsaus E, Montenarh M (1991) Correlation of metabolic stability and altered quaternary structure of oncoprotein p53 with cell transformation. Exp Cell Res 192:157–164
Kuerbitz SJ, Plunkett BS, Walsh WV, Kastan MB (1992) Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci USA 89:7491–7495
Kupryjanczyk J, Thor AD, Beauchamp R, Merrit V, Edgerton SM, Bell DA, Yandell DW (1993) p53 gene mutations and protein accumulation in human ovarian cancer. Proc Natl Acad Sci USA 90:4961–4965
Kupryjanczyk J, Bell DA, Yandell DW, Scully RE, Thor AD (1994) p53 expression in ovarian borderline tumors and stage I carcinomas. Am J Clin Pathol 102:671–676
Lee ET (1980) Statistical methods for survival data analysis. Lifetime Learning, Belmont, Calif
Levine AJ, Momand J, Finlay CA (1991) The p53 tumour suppressor gene. Nature 351:453–456
Marks JR, Davidoff AM, Kerns BJ, Humphrey PA, Pence JC, Dodge RK, Clarke-Pearson DL (1991) Overexpression and mutation of p53 in epithelial ovarian cancer. Cancer Res 51:2979–2984
Mazars R, Pujol P, Maudelonde T, Jeanteur P, Theillet C (1991) p53 mutations in ovarian cancer: a late event? Oncogene 6:1685–1690
Miller C, Mohanadas T, Wolf D, Prokocimer M, Rotter V, Koeffler HP (1986) Human p53 gene localized to short arm of chromosome 17. Nature 319:783–784
Milner BJ, Allan LA, Eccles DM, Kitchener HC, Leonard RCF, Kelly KF, Parkin DE, Haites NE (1993) p53 mutation is a common genetic event in ovarian carcinoma. Cancer Res 53:2128–2132
Naito M, Satake M, Sakai E, Hirano Y, Tsuchida N, Kanzaki H, Ito Y, Mori T (1992) Detection of p53 gene mutations in human ovarian and endometrial cancers by polymerase chain reaction-single strand conformation polymorphism analysis. Jpn J Cancer Res 83:1030–1036
Nigro JM, Baker SJ, Preisinger AC, Jessup JM, Hostetter R, Cleary K, Bigner SH, Davidson N, Baylin S, Devilee P, Glover T, Collins FS, Weston A, Modali R, Harris CC, Vogelstein B (1989) Mutations in the p53 gene occur in diverse human tumour types. Nature 342:705–708
Rasbridge SA, Gillett CE, Seymour A-M, Millis RR (1993) The effect of chemotherapy on histological and biological features of breast carcinoma. J Pathol 169 [Suppl]:191
Soussi T, Caron de Fromentel C, May P (1990) Structural aspects of the p53 protein in relation to gene evolution. Oncogene 5:945–952
Thor AD, Moore DH, Edgerton SM, Kawasaki ES, Reihsaus E, Lynch HT, Marcus JN, Schwartz L, Chen LC, Mayall BH, Smith HS (1992) Accumulation of p53 tumor suppressor gene protein: an independent marker of prognosis in breast cancers. J National Cancer Inst 84:845–855
Vogelstein B, Kinzler KW (1992) p53 function and dysfunction. Cell 70:523–526
Vojtesek B, Bártek J, Midgley CA, Lane DP (1992) An immunochemical analysis of the human nuclear phosphoprotein p53. J Immunol Methods 151:237–244
Younish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimichi A, Oren M (1991) Wild-type p53 induces apoptosis of myeloid leukemic cells that is inhibited by interleukin-6. Nature 352:345–347
Zambetti GP, Bargonetti J, Walker K, Prives C, Levine AJ (1992) Wild-type p53 mediates positive regulation of gene expression through a specific DNA sequence element. Genes Dev 6:1143–1152
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reles, A., Schmider, A., Press, M.F. et al. Immunostaining of p53 protein in ovarian carcinoma: correlation with histopathological data and clinical outcome. J Cancer Res Clin Oncol 122, 489–494 (1996). https://doi.org/10.1007/BF01187161
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01187161