Summary
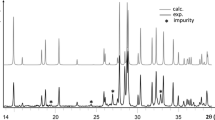
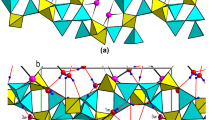
The crystal structure of liebigite, previously only incompletely known from a short note, has been determined from X-ray 4-circle diffractometer data and refined toR=0.030 for 3005 observed reflections. Liebigite from Joachimsthal, Böhmen, was used. It was found to crystallize in the polar orthorhombic space groupBba2−C 172v witha=16.699(3),b=17.557(3),c=13.697(3) Å,V=4016 Å3 and a cell content of 8 Ca2UO2(CO3)3·∼11H2O. The structure contains UO2(CO3)3 units which are linked by two kinds of CaO4(H2O)4 polyhedra and one kind of CaO3(H2O)4 polyhedron to form puckered Ca2UO2(CO3)3·8H2O layers parallel to (010). These layers are interconnected only by hydrogen bonds, both directly as well as via three additional interlayer H2O molecules, two of which show positional disorder.
Zusammenfassung
Die Kristallstruktur des Minerals Liebigit, die bis jetzt nur unzureichend bekannt war, wurde mit Röntgen-Vierkreisdiffraktometer-Daten bestimmt und für 3005 beobachtete Reflexe aufR=0,030 verfeinert. Der untersuchte, von Joachimsthal, Böhmen, stammende Liebigit kristallisiert in der polaren rhombischen RaumgruppeBba2−C 172v mita=16,699(3),b=17,557(3),c=13,697(3) Å,V=4016 Å3 und einem Zellinhalt von 8 Ca2UO2(CO3)3·∼11H2O. Die Struktur enthält UO2(CO3)3-Gruppen, die durch zwei Arten von CaO4(H2O)4-Polyedern und eine Art von CaO3(H2O)4-Polyedern zu buckeligen Ca2UO2(CO3)3·8H2O-Schichten parallel (010) verknüpft sind. Diese Schichten sind nur durch Wasserstoffbrücken verbunden, und zwar sowohl direkt als auch mittels dreier zusätzlicher “freier” Wassermoleküle, von denen zwei eine Lagenfehlordnung aufweisen.
Similar content being viewed by others
References
Alwan, A. K., Williams, P. A., 1980: The aqueous chemistry of uranium minerals. 2. Minerals of the liebigite group. Min. Mag.43, 665–667.
Anderson, A., Chung Chieh, Irish, D. E., Tong, J. P. K., 1980: An X-ray crystallographic. Raman, and infrared spectral study of crystalline potassium uranyl carbonate, K4UO2(CO3)3. Can. J. Chem.58, 1651–1658.
Appleman, D., 1956: Crystal structure of liebigite. Bull. Geol. Soc. Amer. (abstr.)67, 1666.
Čejka, J., 1969: To the chemistry of andersonite and thermal decomposition of dioxotricarbonatouranates. Collection Czechoslov. Chem. Comm.34, 1635–1656.
—,Urbanec, Z., 1976: Uranium secondary minerals in the collections of the National Museum in Prague. IV. Liebigite. (In Czech with English abstract.) Časopis Národního Muzea —oddil přírodovědny145, 1, 26–36.
— 1979: Contribution to the thermal decomposition of liebigite. (In Czech with English abstract.) Časopis Národního Muzea — řada přírodovědná148, 3/4, 177–180.
Coda, A., Della Giusta, A., Tazzoli, V., 1981: The structure of synthetic andersonite, Na2Ca[UO2(CO3)3]·xH2O (x∼5.6). Acta Cryst.B37, 1496–1500.
Dickens, B., Brown, W. E., 1969: The crystal structures of CaNa2(CO3)2·5H2O, synthetic gaylussite, and CaNa2(CO3)2·2H2O, synthetic pirssonite. Inorg. Chem.8, 2093–2103.
Evans, H. T., Jr., Frondel, C., 1950: Studies of uranium minerals (II): Liebigite and uranothallite. Amer. Min.35, 251–254.
Frondel, C., 1958: Systematic Mineralogy of Uranium and Thorium, pp. 108–112. U.S. Geological Survey Bulletin 1064. Washington: U.S. Govt. Print. Office.
Graziani, R., Bombieri, G., Forsellini, E., 1972: Crystal structure of tetraammonium uranyl tricarbonate. J. Chem. Soc. Dalton Trans.1972, 2059–2061.
Kirchheimer, F., 1963: Das Uran und seine Geschichte, pp. 69. Stuttgart: Schweizerbart.
Larsen, E. S., 1917: The probable identity of uranothallite and liebigite. Amer. Min.2, 87.
Mazzi, F., Rinaldi, F., 1961: La struttura cristallina del K3Na(UO2) (CO3)3. Period. Min.30, 1–20.
Meyrowitz, R., 1954: Written communication. In: Systematic Mineralogy of Uranium and Thorium (Frondel, C., 1958), pp. 108–109. U.S. Geological Survey Bulletin 1064. Washington: U.S. Govt. Print. Office.
—,Ross, D. R., Weeks, A. D., 1963: Synthesis of liebigite. U.S. Geological Survey Prof. Paper475B, 162–163.
Sheldrick, G. M., 1976: SHELX, program for crystal structure determination. Univ. of Cambridge.
Walenta, K., 1972: Grimselit, ein neues Kalium-Natrium-Uranylcarbonat aus dem Grimselgebiet (Oberhasli, Kanton Bern, Schweiz). Schweiz. Min. Petr. Mitt.52, 93–108.
Zemann, J., 1981: Zur Stereochemie der Karbonate. Fortschr. Min.59, 1, 95–116.
Author information
Authors and Affiliations
Additional information
With 3 Figures
Rights and permissions
About this article
Cite this article
Mereiter, K. The crystal structure of Liebigite, Ca2UO2(CO3)3·∼11H2O. TMPM Tschermaks Petr. Mitt. 30, 277–288 (1982). https://doi.org/10.1007/BF01087173
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01087173