Abstract
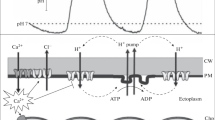
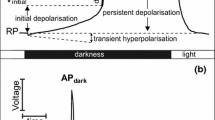
In caulonemal filaments of the mossPhyscomitrella patens (Hedw.), red light triggers a phytochrome-mediated transient depolarisation of the plasma membrane and the formation of side branch initials. Three-electrode voltage clamp and ion flux measurements were employed to elucidate the ionic mechanism and physiological relevance of the red-light-induced changes in ion transport. Current-voltage analyses indicated that ion channels permeable to K+ and Ca2+ are activated at the peak of the depolarisation. Calcium influx evoked by red light coincided with the depolarisation in various conditions, suggesting the involvement of voltage-gated Ca2+ channels. Respective K+ fluxes showed a small initial influx followed by a dramatic transient efflux. A role of anion channels in the depolarising current is suggested by the finding that Cl− efflux was also increased after red light irradiation. In the presence of tetraethylammonium (10 mM) or niflumic acid (1 μM), which block the red-light-induced membrane depolarisation and ion fluxes, the red-light-promoted formation of side branch initials was also abolished. Lanthanum (100 μM), which inhibits K+ fluxes and part of the initial Ca2+ influx activated by red light, reduced the development of side branch initials in red light by 50%. The results suggest a causal link between the red-light-induced ion fluxes and the physiological response. The sequence of events underlying the red-light-triggered membrane potential transient and the role of ion transport in stimulus-response coupling are discussed in terms of a new model for ion-channel interaction at the plasma membrane during signalling.
Similar content being viewed by others
Abbreviations
- [Ca2+]c :
-
cytosolic free Ca2+
- I-V:
-
current-voltage
- E:
-
equilibrium potential
- Pr:
-
red-light-absorbing phytochrome form
- Pr:
-
far-red-light-absorbing phytochrome form
- SPQ:
-
6-methoxy-l-(3-sulphonatopropyl)quinolinium
- TEA:
-
tetraethylammonium
References
Batschauer A, Gilmartin PM, Nagy F, Schäfer E (1994) A molecular approach to photomorphogenesis. In: Kendrick RE, Kronenberg GHM (eds) Photomorphogenesis in plants, 2nd edn. Kluwer Academic Publishers, Dordrecht Boston London, pp 559–599
Beilby MJ (1984) Calcium and plant action potentials. Plant Cell Environ 7: 415–421
Beilby MJ, Coster HGL (1979) The action potential inChara corallina. II. Two activation-inactivation transients in voltage clamps of the plasmalemma. Aust J Plant Physiol 6: 323–335
Bowler C, Chua N-H (1994) Emerging themes of plant signal transduction. Plant Cell 6: 1529–1541
Bowler C, Neuhaus G, Yamagata H, Chua N-H (1994) Cyclic GMP and calcium mediate phytochrome photo-transduction. Cell 77: 73–81
Cove DJ, Knight CD (1993) The mossPhyscomitrella patens, a model system with potential for the study of plant reproduction. Plant Cell 5: 1483–1488
Cove DJ, Schild A, Ashton NW, Hartmann E (1978) Genetic and physiological studies of the effect of light on the development of the mossPhyscomitrella patens. Photochem Photobiol 27: 249–254
Elzenga JTM, Prins HBA, Van Volkenburgh E (1995) Light-induced membrane potential changes of epidermal and mesophyll cells in growing leaves ofPisum sativum. Planta 197: 127–134
Erhardt DW, Atkinson EM, Long SR (1992) Depolarization of alfalfa root hair membrane potential byRhizobium meliloti Nod factors. Science 256: 998–1000
Ermolayeva E, Hohmeyer H, Johannes E, Sanders D (1996) Calcium-dependent membrane depolarisation activated by phytochrome in the mossPhyscomitrella patens. Planta 199: 352–358
Felle HH, Kondorosi E, Kondorosi A, Schultz M (1995) Nod signal-induced plasma-membrane potential changes in alfalfa root hairs are differentially sensitive to structural modifications of the lipooligosaccharide. Plant J 7: 939–947
Finn JT, Grunwald ME, Yau KW (1996) Cyclic nucleotide-gated ion channels — an extended family with diverse functions. Annu Rev Physiol 58: 395–426
Gradmann D, Hansen U-P, Long WS, Slayman CL, Warncke J (1978) Current-voltage relationships for the plasma membrane and its principal electrogenic pump inNeurospora crassa. I. Steady-state conditions. J Membr Biol 39: 333–367
Grimsley NH, Featherstone DR, Courtice GRM, Ashton NW, Cove DJ (1979) Somatic hybridization following protoplast fusion as a tool for the analysis of development in the moss,Physcomitrella patens. In: Advances in protoplast research. Proceedings of the 5th International Protoplast Symposium, Szeged, Hungary, Academiai Kiado, Budapest, Hungary, pp 363–376
Haley A, Russell AJ, Wood N, Allan AC, Knight M, Campbell AK, Trewavas AJ (1995) Effects of mechanical signaling on plant cell cytosolic calcium. Proc Natl Acad Sci USA 92: 4124–4128
Hartmann E, Jenkins GI (1984) Photomorphogenesis of mosses and liverworts. In: Dyer AF, Duckett JG (eds) Experimental biology of bryophytes. Academic Press, London, pp 203–228
Hepler PK, Wayne RO (1985) Calcium and plant development. Annu Rev Plant Physiol 36: 397–439
Hoshi T (1995) Regulation of voltage dependence of the KAT1 channel by intracellular factors. J Gen Physiol 105: 309–328
Kasamo K (1981) Effect of abscisic acid on the K+ efflux and membrane potential ofNicotiana tabacum leaf cells. Plant Cell Physiol 22: 1257–1267
Kendrick RE, Kronenberg GHM (1994) Photomorphogenesis in plants, 2nd edn. Kluwer Academic Publishers, Dordrecht Boston London
Marten I, Zeilinger C, Redhead C, Landry DW, Al-Awagati Q, Hedrich R (1992) Identification and modulation of a voltage-dependent anion channel in the plasma membrane of guard cells by high affinity ligands. EMBO J 11: 3569–3575
Mathieu Y, Kurkdjian A, Xia H, Guern J, Koller A, Spiro MD, O'Neill M, Albersheim P, Darvill A (1991) Membrane responses induced by oligogalacturonides in suspension-cultured tobacco cells. Plant J 1: 333–343
Moyen C, Johannes E (1996) Systemin transiently depolarizes the tomato mesophyll cell membrane and antagonizes fusicoccininduced extracellular acidification of mesophyll tissue. Plant Cell Environ 19: 464–470
Neuhaus G, Bowler C, Kern R, Chua N-H (1993) Calcium/calmodulin-dependent and -independent phytochrome signal transduction pathways. Cell 73: 937–952
Pelissier B, Thibaud JB, Grignon C, Esquerré-Tugayé MT (1986) Cell surfaces in plant-microorganism interactions. VII. Elicitor preparations from two fungal pathogens depolarize plant membranes. Plant Sci 46: 103–109
Pope AJ, Leigh RA (1988) The use of a chloride-sensitive fluorescent probe to measure chloride transport in isolated tonoplast vesicles. Planta 176: 451–460
Quail PH, Boylan MT, Parks BM, Short TW, Xu Y, Wagner D (1995) Phytochromes: photosensory perception and signal transduction. Science 268: 675–680
Racusen RH, Cooke T (1982) Electrical changes in the apical cell of the fern gametophyte during irradiation with photomorphogenetically active light. Plant Physiol 70: 331–334
Racusen RH, Satter RL (1975) Rhythmic and phytochromeregulated changes in transmembrane potential inSamanea pulvini. Nature 255: 408–410
Roux SJ (1994) Signal transduction in phytochrome responses. In: Kendrick RE, Kronenberg GHM (eds) Photomorphogenesis in plants, 2nd edn. Kluwer Academic Publishers, Dordrecht Boston London, pp 187–209
Schroeder JI, Hagiwara S (1989) Cytosolic calcium regulates ion channels in the plasma membrane ofVicia faba guard cells. Nature 338: 427–430
Schroeder JI, Schmidt C, Sheaffer J (1993) Identification of highaffinity slow anion channel blockers and evidence for stomatal regulation by slow anion channels in guard cells. Plant Cell 5: 1831–1841
Schumaker KS, Gizinski MJ (1993) Cytokinin stimulates dihydropyridine-sensitive calcium uptake in moss protoplasts. Proc Natl Acad Sci USA 90: 10937–10941
Schumaker KS, Gizinski MJ (1995) 1,4-Dihydropyridine binding sites in moss plasma membranes. J Biol Chem 270: 23461–23467
Schwartz A, Ilan N, Schwartz M, Scheaffer J, Assmann SM, Schroeder JI (1995) Anion-channel blockers inhibit S-type anion channels and abscisic acid responses in guard cells. Plant Physiol 109: 651–658
Shacklock PS, Read ND, Trewavas AJ (1992) Cytosolic free calcium mediates red light-induced photomorphogenesis. Nature 358: 753–755
Spalding EP, Cosgrove DJ (1989) Large plasma-membrane depolarization precedes rapid blue-light-induced growth inhibition in cucumber. Planta 178: 407–410
Spalding EP, Cosgrove DJ (1992) Mechanism of blue-light-induced plasma membrane depolarization in etiolated cucumber hypocotyls. Planta 188: 199–205
Tester M (1990) Plant ion channels: whole-cell and single-channel studies. New Phytol 114: 305–340
Tester M, MacRobbie EAC (1990) Cytoplasmic calcium affects the gating of potassium channels in the plasma membrane ofCharacorallina: a whole-cell study using calcium-channel effectors. Planta 180: 569–581
Thain JF, Doherty HM, Bowles DJ, Wildon DC (1990) Oligosaccharides that induce proteinase inhibitor activity in tomato plants cause depolarization of tomato leaf cells. Plant Cell Environ 13: 569–574
Thain JF, Gubb IR, Wildon DC (1995) Depolarization of tomato leaf cells by oligogalacturonide elicitors. Plant Cell Environ 18: 211–214
Thiel G, MacRobbie EAC, Blatt MR (1992) Membrane transport in stomatal guard cells: the importance of voltage control. J Membr Biol 126: 1–18
Trebaçz K, Simonis W, Schönknecht G (1994) Cytoplasmic Ca2+, K+, Cl− and NO3- activities in the liverwortConocephalum conicum L. at rest and during action potentials. Plant Physiol 106: 1073–1084
Tyerman SD (1992) Anion channels in plants. Annu Rev Plant Physiol Plant Mol Biol 43: 351–373
Ward JM, Pei Z-M, Schroeder JI (1995) Roles of ion channels in initiation of signal transduction in higher plants. Plant Cell 7: 833–844
Weisenseel MH, Ruppert HK (1977) Phytochrome and calcium ions are involved in light-induced membrane depolarization inNitella. Planta 137: 225–229
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ermolayeva, E., Sanders, D. & Johannes, E. Ionic mechanism and role of phytochrome-mediated membrane depolarisation in caulonemal side branch initial formation in the mossPhyscomitrella patens . Planta 201, 109–118 (1997). https://doi.org/10.1007/BF01007695
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01007695