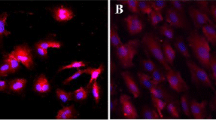
Abstract
The exertion of periodic dynamic strain on the arterial wall is hypothesized to be relevant to smooth muscle cell morphology and function. This study has investigated the effect of cyclic mechanical stretching on rabbit aortic smooth muscle cell proliferation and expression of contractile phenotype protein markers. Cells were cultured on flexible-bottomed dishes and cyclic stretch was applied (frequency 30 cycles/min, 15% elongation) using a Flexercell Strain unit. Cyclic stretch potentiated smooth muscle cell proliferation in serum-activated cultures but not in cultures maintained in 0.5% fetal calf serum. Stretching induced a serum-independent increase of h-caldesmon expression and this effect was reversible following termination of mechanical stimulation. Strain was without effect on smooth muscle myosin or calponin expression. In cells grown on laminin stretch-induced h-caldesmon expression was more prominent than in cells cultured on collagen types I and IV, poly-L-lysine and gelatin. These data suggest that cyclic mechanical stimulation possesses dual effect on vascular smooth muscle cell phenotype characteristics since it: 1) potentiates proliferation, an attribute of a dedifferentiated phenotype; and 2) increases expression of h-caldesmon considered a marker of a differentiated smooth muscle cell state.
Similar content being viewed by others
References
Barany K, Ledvora RF, Mougios V, Barany M: Stretch-induced myosin light chain phosphorylation and stretch-release-induced tension development in arterial smooth muscle. J Biol Chem 260: 7126–7130, 1985
Schwartz SM, Campbell GR, Campbell JH: Replication of smooth muscle cells in vasculardisease. Circ Res 58: 427–444, 1986
Campbell GR, Campbell JH, Manderson JA, Horrigan S, Rennick RE: Arterial smooth muscle. A multifunctional mesenchymal cell. Arch Pathol Lab Med 112: 977–986, 1988
Mosse PRL, Campbell GR, Wang ZL, Campbell JH: Smooth muscle phenotypic expression in human carotid arteries. I. Comparison of cells from diffuse intimal thickenings adjacent to atheromatous plaques with those of the media. Lab Invest 53: 556–562, 1985
Gabbiani g, Kocher O, Bloom WS, Vandekerckhove J, Weber K: Actin expression in smooth muscle cells of rat aortic intimal thickening, human atheromatous plaque, and cultured rat aortc media. J Clin Invest 73: 148–152, 1984
Kocher O, Skalli O, Bloom WS, Gabbiani G: Cytoskeleton of rat aortic smooth muscle cells. Normal conditions and experimental intimal thickening. Lab Invest 50: 645–652, 1984
Zanellato AMA, Borrione AC, Tonello M, Scannapieco G, Pauletto P, Sartore S: Myosin isoform expression and smooth muscle cell heterogeneity in normal and atherosclerotic rabbit aorta. Arteriosclerosis 10: 996–1009, 1990
Glukhova MA, Kabakov AE, Frid MG, Ornatsky OI, Belkin AM, Mukhin DN, Orekhov AN, Koteliansky VE, Smirnov VN: Modulation of human aorta smooth muscle cell phenotype: a study of muscle specific variants of vinculin, caldesmon, and actin expression. Proc Natl Acad Sci USA 85: 9542–9546, 1988
Sadoshima J, Jahn L, Takahashi T, Kulik T, Izumo S: Molecular characterization of the stretch-induced adaptation of cultured cardiac cells. J Biol Chem 267: 10551–10560, 1992
Comuro I, Katoh Y, Toshikazu K, Shibazaki Y, Kurabayashi M, Hoh E, Takaku F, Yazaki Y: Mechanical loading stimulates cell hypertrophy and specific gene expression in cultured rat cardiac myocytes. J Biol Chem 266: 1265–1268, 1991
Gardner DG, Wirtz H, Dobbs LG: Stretch-dependent regulation of atrial peptide synthesis and secretion in cultured atrial cardiocytes. Am J Physiol 263: E239-E244, 1992
Sumpio BE, Banes AJ: Prostacyclin synthetic activity in cultured aortic endothelial cells undergoing cyclic mechanical deformation. Surgery 104: 383–389, 1988
Iba T, Shin T, Sonoda T, Rosales O, Sumpio BE: Stimulation of endothelial secretion of tissue-type plasminogen activator by repetitive stretch. J Surg Res 50: 457–460, 1991
Sumpio BE, Banes AJ, Levin LG, Johnson G: Mechanical stress stimulates aortic endothelial cells to proliferate. J Vasc Surg 6: 252–256, 1987
Leung DY, Glagov S, Mathews MB: Cyclic stretching stimulates synthesis of matrix components by arterial smooth muscle cellsin vitro. Science (Wash. DC) 191: 475–477, 1976
Wilson E, Mai Q, Sudhir K, Weiss RH, Ives HE: Mechanical strain induces growth of Vascular smooth muscle cells via autocrine action of PDGF. J Cell Biol 123: 741–747, 1993
Hayashi K, Yano H, Hashida T, Takeuchi R, Takeda O, Asada K, Takahashi E, Kato I, Sobue K: Genomic structure of the human caldesmon gene. Proc Natl Acad Sci USA 89: 12122–12126, 1992
Makuch R, Birukov K, Shirinsky V, Dabrowska R: Functional interrelationship between calponin and caldesmon. Biochem J 280: 33–38, 1991
Gimona M, Herzog M, Vandekerckhove J, Small JV: Smooth muscle specific expression of calponin. FEBS Lett 274: 159–162, 1990
Birukov KG, Frid MG, Rogers JD, Shirinsky VP, Koteliansky VE, Campbell JH, Campbell GR: Synthesis and expression of smooth muscle phenotypmarkers in primary culture of rabbit smooth muscle cells: influence of seeding density and media and relation to cell contractility. Exp Cell Res 204: 46–53, 1993
Chamley-Campbell JH, Campbell GR, Ross R: The smooth muscle cell in culture. Physiol Rev 59: 1–61, 1979
Shirinsky VP, Antonov AS, Birukov KG, Sobolevsky AV, Romanov YuA, Kabaeva NV, Antonova GN, Smirnov VN: Mechano-chemical control of human endothelium orientation and size. J Cell Biol 109: 331–339, 1989
Shirinsky VP, Biryukov KG, Vorotnikov AV, Gusev NB: Caldesmon 150, caldesmon 77 and skeletal muscle troponin T share a common antigenic determinant. FEBS Lett 251: 65–68, 1989
Birukov KG, Stepanova OV, Nanev AK, Shirinsky VP: Expression of calponin in rabbit nd human aortic smooth muscle cells. Cell Tis Res 266: 579–584, 1991
Nanaev AK, Shirinsky VP, Birukov KG: Immunofluorescent study of heterogeneity in smooth muscle cells of human fetal vessels using antibodies to myosin, desmin, and vimentin. Cell Tis Res 266: 535–540, 1991
Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970
Towbin H, Staehelin T, Gordon J: Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76: 4350–4354, 1979
Shirinsky VP, Birukov KG, Koteliansky VE, Glukhova MA, Spanidis E, Rogers JD, Campbell JH, Campbell GR: Density-related expression of caldesmon and vinculin in cultured rabbit aortic smooth muscle cells. Exp Cell Res 194: 186–189, 1991
Shirinsky VP, Birukov KG, Sobolevsky AV, Vedernikov YuP, Posin EYa, Popov EG: Contractile rabbit aortic smooth muscle cells in culture. Preparation and characterization. Am J Hypertension 5: 124S-130S, 1992
Holycross BJ, Blank RS, Thompson MM, Peach MJ, Owens GK: Platelet-derived growth factor-BB-induced suppression of smooth muscle cell differentiation. Circ Res 71: 1525–1532, 1992
Yamamoto M, Yamamoto K, Noumura T: Type I collagen promotes modulation of cultured rabbit arterial smooth muscle cells from a contractile to a synthetic phenotype. Exp Cell Res 204: 121–129, 1993
Hedin U, Bottger BA, Forsberg E, Johansson S, Thyberg J: Diverse effects of fibronectin and laminin on phenotypic properties of cultured arterial smooth muscle cells. J Cell Biol 107: 307–319, 1988
Schaller MD, Parsons JT: Focal adhesion kinase: an integrin-linked protein tyrosine kinase. Trends Cell Biol 3: 258–262, 1993
Kornberg LJ, Earp HS, Turner CE, Prockop C, Juliano RL: Signal transduction by integrins: increased protein tyrosine phosphorylation caused by clustering of beta 1 integrins. Proc Natl Acad Sci USA 88: 8392–8396, 1991
Seifert RA, Schwartz SM, Bowen-Pope DF: Developmentally regulated production of platelet-derived growth factor-like molecules. Nature 311: 669–671, 1984
Morisaki N, Kanzaki T, Koshikawa T, Saito Y, Yoshida S: Secretion of a new growth factor, smooth muscle cell derived growth factor, distinct from platelet derived growth factor by cultured rabbi aortic smooth muscle cells. FEBS Lett 230: 186–190, 1988
Naruse K, Sokabe M: Involvement of stretch-activated ion channels in Ca2+ mobilization to mechanical stretch in endothelial cells. Am J Physiol 264: C1037-C1044, 1993
Berk BC, Alexander RW, Brock TA, Gimbrone MA Jr, Webb RC: Vasoconstriction: a new activity for platelet-derived growth factor. Science 232: 87–90, 1986
Berk BC, Alexander RW: Vasoactive effects of growth factors. Biochem Pharmacol 38: 219–225, 1989
Nilsson J, Sjolund M, Palmberg L, Von Euler AM, Jonzon B, Thyberg J: The calcium antagonist nifedipine inhibits arterial smooth muscle cell proliferation. Atherosclerosis 58: 109–122, 1985
Corjay MH, Thompson MM, Lynch KR, Owens GK: Differential effect of platelet-derived growth factor versus serum-induced growth on smooth muscle alpha-actin and nonmuscle beta-actin mRNA expression and growth state in cultured rat aortic smooth muscle cells. J Biol Chem 264: 10501–10506, 1989
Desmouliere A, Rubbia-Brandt L, Gabbiani G: Modulation of actin isoform expression in cultured arterial smooth muscle cells by heparin and culture conditions. Arteriosclerosis and Thrombosis 11: 244–253, 1991
Ueki N, Sobue K, Kanda K, Hada T, Higashino K: Expression of high and low molecular weight caldesmons during phenotypic modulation of smooth muscle cells. Proc Natl Acad Sci USA 84: 9049–9053, 1987
Reckless J, Fleetwood G, Tilling L, Huber PAJ, Marston SB, Pritchard K: Changes in the caldesmon isoform content and intimal thickening in the rabbit carotid artery induced by a silicone elastomer collar. Arterioscler Thromb 14: 1837–1845, 1994
McDonald JA: Receptors for extracellular matrix components. Am J Physiol 257: L331-L337, 1989
Davis MJ, Donovitz JA, Hood JD: Stretch-activated single-channel and whole cell currents in vascular smooth muscle cells. Am J Physiol 262: C1083-C1088, 1992
Bialecki RA, Kulik TJ, Colucci WS: Stretching increases calcium influx and efflux in cultured pulmonary arterial smooth muscle cells. Am J Physiol 263: L602-L606, 1992
Kulik TJ, Bialecki RA, Colucci WS, Rothmann A, Glennon T, Underwood RH: Stretch increases inositol trisphosphate and inositol tetrakisphosphate in cultured pulmonary vascular smooth muscle cells. Biochem Biophys Res Commun 180: 983–987, 1991
Letsou GV, Rosales O, Maitz S, Vogt A, Sumpio BE: Stimulation of adenylate cyclase activity in cultured endothelial cells subjected to cyclic stretch. J Cardiovasc Surg 31: 634–639, 1990
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Birukov, K.G., Shirinsky, V.P., Stepanova, O.V. et al. Stretch affects phenotype and proliferation of vascular smooth muscle cells. Mol Cell Biochem 144, 131–139 (1995). https://doi.org/10.1007/BF00944392
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00944392