Conclusions
-
1.
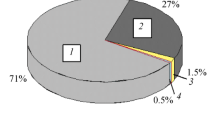
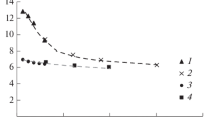
Naphthalene can be hydrogenated (mainly to tetralin) to the extent of 75–80% on hydrogenating it at a hydrogen pressure of 20 atm, a temperature of 350–370dgC, and a crude feed of 0.25–1.0 kg/liter · h, on using Al2O3 + CoO + MoO3 catalyst. The hydrogenation conversion level of 2-methylnaphthalene is 1.5 times less. Naphthalene can be hydrogenated to the extent of 90–95% at a pressure of 40 atm on using a tungsten— nickel—sulfide catalyst. A significant fraction of the tetralin formed is, however, hydrogenated further to the decalins.
-
2.
Naphthalene can be converted to the extent of 75–80% into tetralin and decalin on hydrogenating naphthalene fractions containing 42–76% naphthalene at 350° C, under a pressure of 40 atm, and crude feed of 0.3 kg/liter · h. Decalin formation can be reduced by cutting down the hydrogen pressure to 20 atm. To obtain a hydrogenizate which is suitable as a crude for isolating tetralin by narrow cut fractionation, the naphthalene concentration of the crude should be greater than 60% and that of the tetralin in the hydrogenizate should not be less than 45–50%.
-
3.
By using a column with an efficiency of 20 theoretical plates it is possible to recover 80% of the potential amount of tetralin, in terms of the naphthalene present in the crude, from the hydrogenizate obtained at a pressure of 40 atm and which contains about 75% of tetralin and decalin. Decalin in amounts of 10–12% is obtained at the same time. By fractionating a hydrogenizate obtained at a lower pressure, the yield of tetralin can be increased to 90–95% in terms of the naphthalene present in the crude to be hydrogenated.
Similar content being viewed by others
Literature cited
N. V. Shavolina, D. F. Kasatkin, and V. I. Karzhev, Koks i Khimiya, No. 1 (1959).
R. J. Hogan, K. L. Mills, and W. C. Zanntng, J. Chem. and Eng.,7, No. 1 (1958).
I. I. Eru, A. A. Lange, V. I. Timoshenko, and E. T. Kir'yanova, Koks i Khimiya, No. 5 (1961).
T. P. Wilson, F. G. Caffisch, and F. M. Leo, J. Phys. Chem.,62, 1069 (1958).
A. A. Krichko, in: Chemical Processing of Fuels [in Russian], Izd. “Nauka,” (1965), p. 135; Trudy IGI,24, No. 3, Izd. “Nedra” (1967).
A. A. Krichko, D. V. Skvortsov, L. S. Sovetova, and B. S. Filippov, Trudy IGI,23, No. 4, Izd. “Nedra” (1968).
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 1, pp. 14–17, January, 1969.
Rights and permissions
About this article
Cite this article
Krichko, A.A., Skvortsov, D.V., Titova, T.A. et al. Production of tetralin by the hydrogenation of naphthalene-containing fractions. Chem Technol Fuels Oils 5, 18–22 (1969). https://doi.org/10.1007/BF00727949
Issue Date:
DOI: https://doi.org/10.1007/BF00727949