Abstract
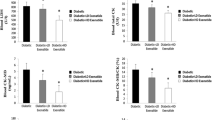
The aim of the present experimental study in the rat heart was to assess cardiac performance and metabolism in mild diabetes of 2 months' duration (postprandial blood sugar levels of 307±101 mg/dl and nearly normal fasting blood glucose of 102±40 mg/dl) using the working rat heart model at physiological workload with a perfusion time of 60 min. We also compared the effect of two forms of therapy for diabetes, islet transplantation and insulin therapy (s.c.), after 2 months. A 36% reduction in glucose utilization is metabolically characteristic for the diabetic heart, mainly caused by a 55% reduced glucose uptake (P<0.001), but also by a nearly twofold increased lactate and pyruvate production (P<0.001). This reduced carbohydrate metabolism is accompanied by a 37% reduction of oxygen uptake (P<0.001) as well as a significant reduction in myocardial ATP and CP levels (P<0.001), resulting in a significantly reduced cardiac output (P<0.001). Moreover, the balance of energy reveals that the diabetic heart obtains 46% of its energy requirements for 1 h from endogenous glycogen, whereas the control heart obtains 91% of its energy needs (i.e. preferentially) from exogenous glucose (only 9% from endogenous glycogen). Both investigated therapeutic interventions led to a complete reversibility of the hemodynamic and metabolic alterations, indicating that the cause of diabetic cardiomyopathy in this model of mild and short-term diabetes is due to a defect in cardiac carbohydrate metabolism, which is correctable by insulin administration.
Similar content being viewed by others
References
Allard MF, Schönekess BO, Henning SL, English DR, Lopaschuk GD, Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol 267:H742-H750, 1994
Bergmeyer HU, Methoden der enzymatischen Analyse Verlag Chemie, Weinheim, 1974
Bretzel RG, Hering BJ, Linn T, Strödter D, Wiegand S, Woehrle M, Zekorn T, Islet transplantation in rodents. In: Bretzel RG (ed), Diabetes mellitus. Immunological and dynamic aspects of insulin substitution. Springer, Berlin Heidelberg New York, pp 229–252, 1990
Chatham JC, Forder JR, A13C-NMR study of glucose oxidation in the intact functioning rat heart following diabetes-induced cardiomyopathy. J Mol Cell Cardiol 25:1203–1213, 1993
Denton RM, Randle PJ, Concentrations of glycerides and phospholipids in rat heart and gastrocnemius muscles. Biochem J 104:416–422, 1967
Eggstein M, Kreutz FH, Eine neue Bestimmung der Neutralfette im Blutserum und Gewebe. Klin Wochenschr 44: 262–273, 1966
Gamble J, Lopaschuk GD, Glycolysis and glucose oxidation during reperfusion of ischemic heart from diabetic rats. Biochim Biophys Acta 1225:191–199, 1994
Garvey GT, Hardin D, Juhaszova M, Dominguez JH, Effects of diabetes on myocardial glucose transport system in rats: implications for diabetic cardiomyopathy. Am J Physiol 264: H837-H844, 1993
Jackson CV, McGrath GM, Tahiliani AG, Vadlamudi RVSV, McNeill JH, A functional and ultrastructural analysis of experimental rat myocardium. Diabetes 34:876–883, 1985
Kainulainen H, Schurmann A, Vilja P, Joost HG, In-vivo glucose uptake and glucose transporter proteins GLUT1 and GLUT3 in brain tissue from streptozotocin-diabetic rats. Acta Physiol Scand 149:221–225, 1993
Kainulainen H, Breiner M, Schurmann A, Marttinen A, Virjo A, Joost HG, In vivo glucose uptake and glucose transporter proteins GLUT1 and GLUT4 in heart and various types of skeletal muscle from streptozotocin-diabetic rats. Biochim Biophys Acta 1225:275–282, 1994
Keppler D, Decker K, Glykogen: Bestimmung mit Amyloglu-cosidase. In: Bergmeyer HU (ed) Methoden der enzymatischen Analyse, Vol II. Verlag Chemie, Weinheim, pp 1171–1176, 1974
Kerbey AL, Radcliffe PM, Randle PJ, Diabetes and the control for pyruvate dehydrogenase in rat heart mitochondria by concentration ratios of adenosine triphosphate/adenosine diphosphate, of reduced/oxidised nicotinamide-adenine dinucleotide and of acetylcoenzyme A/coenzyme A. Biochem J 164: 509–519, 1977
Kobayashi K, Neely JR, Control of maximum rates of glycolysis in rat cardiac muscle. Circ Res 44:166–175, 1979
Kobayashi K, Neely JR, Effects of increased cardiac work on pyruvate-dehydrogenase activity in hearts from diabetic animals. J Mol Cell Cardiol 15:347–357, 1983
Lamprecht W, Trautschold I, Adenosin-5′-triphosphat. In: Bergmer HU (ed) Methoden der enzymatischen Analyse, Vol II. Verlag Chemie, Weinheim, pp 1251–1259, 1974
Lamprecht W, Stein P, Heinz F, Weisser H, Creatinphosphat. In: Bergmeyer HU (ed) Methoden der enzymatischen Analyse, Vol II. Verlag Chemie, Weinheim, pp 1729–1733, 1974
Lopaschuk GD, Spafford M, Response of isolated working hearts to fatty acids and carnitine palmitoyltransferase I inhibition during reduction of coronary flow in acutely and chronically diabetic rats. Circ Res 65:378–387, 1989
Lopaschuk GD, Spafford MA, Energy substrate utilization by isolated working hearts from newborn rabbits. Am J Physiol 258:H1274-H1280, 1990
Lopaschuk GD, Tsang H, Metabolism of palmitate in isolated working hearts from spontaneously diabetic “BB” Wistar rats. Circ Res 61:853–858, 1987
Murthy KV, Shipp JC, Heart triglyceride synthesis in diabetes: selective increase in activity of enzymes of phosphatidate synthesis. J Mol Cell Cardiol 12:299–309, 1980
Neely JR, Liebermeister H, Battersby EJ, Morgan HE, Effect of pressure development on oxygen consumption by isolated rat heart. Am J Physiol 212:804–814,1967
Neely JR, Denton RM, England PJ, Randle PJ, The effects of increased heart work on the tricarboxylate cycle and its interactions with glycolysis in the perfused rat heart. Biochem J 128: 147–159, 1972
Nicholl TA, Lopaschuk GD, McNeill JH, Effects of free fatty acids and dichloroacetate on isolated working diabetic rat heart. Am J Physiol 261:H1053-H1059, 1991
Penpargkul S, Schaible T, Yipinstoi T, Scheuer J, The effect of diabetes on performance and metabolism of rat hearts. Circ Res 47:911–927, 1980
Pieper GM, Murray WJ, Salhany JM, Wu ST, Eliott TS, Salient effects ofL-carnitine on adenine-nucleotide loss of coenzyme A acylation in the diabetic heart perfused with excess palmitic acid A phosphorous-31 NMR and chemical extract study. Biochim Biophys Acta 803:229–240, 1984
Postic C, Leturgue A, Printz RL, Maulard P, Loizeau M, Granner DK, Girard J. Development and regulation of glucose transporter and hexokinase expression in rat. Am J Physiol 266: E548-E559, 1994
Puckett SW, Reddy WJ, A decrease in the malate-aspartate-shuttle and glutamate translocase activity in heart mitochondria from alloxan-diabetic rats. J Mol Cell Cardiol 11:173–187, 1979
Reinilä A, Akerblom HK, Ultrastructure of heart muscle in short-term diabetic rats: influence of insulin treatment. Diabetologia 27:397–402, 1984
Rösen P, Adrian M, Feuerstein J, Reinauer H, Glycolysis and glucose oxidation in the rat heart under nonrecirculating perfusion conditions. Basic Res Cardiol 79:307–312, 1984
Rösen P, Windeck P, Zimmer HG, Frentel H, Bürring KF, Reinauer H, Myocardial performance and metabolism in non-ketotic diabetic rats: myocardial function and metabolism in vivo and the isolated perfused heart under the influence of insulin and octanoate. Basic Res Cardiol 81:620–635, 1986
Stroedter D, Willmann P, Willmann J, Federlin K, Schaper W, Results of a balance of energy in the diabetic heart. In: Nagano M, Dhalla NS (eds) The diabetic heart. Raven Press, New York, pp 383–393, 1991
Stroedter D, Mahler M, Bretzel RG, Federlin K, Long-term therapy with islet transplantation is more effective with regard to the reversibility of diabetes-induced hemodynamic and metabolic cardiac alterations. Transplant Proc 26:672, 1994
Sudgen PH, Smith DM, The effects of insulin on glucose uptake and lactate release in perfused working rat heart preparations. Biochem J 206:473–479, 1982
Taegtmeyer H, Passmore JM, Defective energy metabolism of the heart in diabetes. Lancet 19:139–141, 1985
Taegtmeyer H, Hems R, Krebs HA, Utilisation of energy-providing substrates in the isolated working rat heart. Biochem J 186:701–711, 1980
Tahiliani AG, McNeill JH, Diabetes induced abnormalities in the myocardium. Life Sci 38:959–974, 1986
Tahiliani AG, Lopaschuk GD, McNeill JH, Effect of insulin treatment on long-term diabetes-induced alteration of myocardial function. Gen Pharmacol 15:545–547,1984
Wall SR, Lopaschuk GD, Glucose oxidation rates in fatty-acid perfused isolated working hearts from diabetic rats. Biochim Biophys Acta 1006:97–103, 1989
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stroedter, D., Schmidt, T., Bretzel, R.G. et al. Glucose metabolism and left ventricular dysfunction are normalized by insulin and islet transplantation in mild diabetes in the rat. Acta Diabetol 32, 235–243 (1995). https://doi.org/10.1007/BF00576256
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00576256