Summary
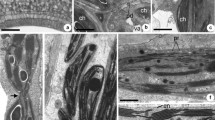
Electron micrographs of the zone of separation in flower pedicels of Lycopersicon esculentum and Nicotiana tabacum are presented with particular reference to the indentation of epidermal tissue in the abscission zone, subcellular organelles, and the cell wall. The indentation or groove which delineates the abscission zone extends some distance into the pedicel with branchings off the main groove. These branches are approximately 20 mμ in width while the main groove averages approximately 200 mμ in width. Invaginations of the plasmalemma are observed with considerable frequency. within these invaginations one observes a material of about the same density as the cell wall except that it is more fibrillar. Plasmodesmata are also observed, with considerable branching into middle lamellae of cells comprising the abscission zones. Microbodies with crystalloid cores appear with considerable frequency in cells of the abscission zone. The crystalloids appear to be cubical in shape and are composed of parallel sheets of osmiophilic material. The sheets average about 6 mμ in thickness and are spaced at 4 mμ intervals. The microbodies with crystalloid cores are observed to be characteristically of two size groupings. In tobacco the microbodies average 900 mμ and 1,500 mμ in profile. In tomato they average 300 mμ and 500 mμ. Chloroplasts contain a granular component which is membrane-enclosed. The component is large in comparison with the plastid in which it occurs, averaging 1.2–1.4 μ in diameter in chloroplasts ranging from 1.6 μ to 2.2 μ in diameter. The inner membrane of the chloroplast is highly invaginated, and DNA- and phytoferritin-like materials are observed within the plastids. Microtubules with an average diameter of 20 mμ are observed adjacent and parallel to the plasmalemma, primarily in the corners of the cells. Micrographs of other normally occurring cytoplasmic inclusions are also presented.
Similar content being viewed by others
References
Addicott, F. T., and R. S. Lynch: Physiology of abscission. Ann. Rev. Plant Physiol. 6, 211–238 (1955).
Bisalputra, T., and A. Bisalputra: The occurrence of DNA fibrils in chloroplasts of Laurencia spectabilis. J. Ultrastruct. Res. 17, 14–22 (1967).
Bornman, C. H., A. R. Spurr, and F. T. Addicott: Abscisin, auxin, and gibberellin effects on the developmental aspects of abscission in cotton (Gossypium hirsutum). Amer. J. Bot. 54, 125–135 (1967).
Cronshaw, J.: Crystal containing bodies of plant cells. Protoplasma (Wien) 39, 318–325 (1964).
Duve, C. De: Intracellular localization of enzymes. Nature (Lond.) 187, 836–838 (1960).
Esau, K., V. I. Cheadle, and R. H. Gill: Cytology of differentiating tracheary elements. II. Structures associated with cell surfaces. Amer. J. Bot. 53, 765 bis 771 (1966).
Girola, F. M., e G. Dassu: L'evoluzione dei chloroplasti durante l'inverdimento sperimentale di frammenti di tuberi di topenambour (Helianthus tuberosus). Nuovo G. bot. ital. 67, 63–78 (1960).
Israel, H. W., and F. C. Steward: The fine structure and development of plastids in cultured cells of Daucus carota. Ann. Bot. 31, 1–18 (1967).
Kislev, N., H. Swiet, and L. Bogorad: Nucleic acids of chloroplasts and mitochondria in swiss chard. J. Cell Biol. 25, 327–344 (1965).
Ledbetter, M. C., and K. R. Porter: A “microtubule” in plant cell fine structure. J. Cell Biol. 19, 239–250 (1963).
Leinweber, C. L., and W. C. Hall: Foliar abscission in cotton. III. Macroscopic and microscopic changes associated with natural and chemically induced leaf fall. Bot. Gaz. (Chicago) 121, 9–16 (1959)
Luft, J. H.: Improvements in epoxy resin embedding methods. J. biophys. biochem. Cytol. 9, 409–414 (1961).
Marinos, N. G.: Multifunctional plastids in the meristimatic region of potato tuber buds. J. Ultrastruct. Res. 17, 91–113 (1967).
Murmanis, L., and R. F. Evert: Parenchyma cells of secondary phloem in Pinus strobus. Planta (Berl.) 73, 301–318 (1967).
Newcomb, E. H.: Fine structure of protein-storing plastids in bean root tips. J. Cell Biol. 33, 143–163 (1967).
Srivastava, L. M.: On the fine structure of the cambium of Fraxinus americana L. J. Cell Biol. 31, 79–93 (1966).
Thornton, R. M., and K. V. Thimann: On a crystal-containing body in cells of the oat coleoptile. J. Cell Biol. 20, 345–350 (1964).
Whaley, W. G., and H. H. Mollenhauer: The Golgi apparatus and cell plate formation — a postulate. J. Cell Biol. 17, 216–221 (1963).
Yager, R. E.: The mechanism of floral abscission in Nicotiana. Doct. Diss. Univ. of Iowa, Iowa City 1957.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jensen, T.E., Valdovinos, J.G. Fine structure of abscission zones. Planta 77, 298–318 (1967). https://doi.org/10.1007/BF00389317
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00389317