Abstract
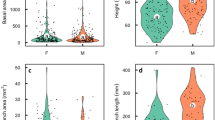
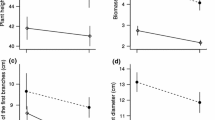
We investigated the role of photosynthesis by reproductive organs in meeting the carbon costs of sexual reproduction in the snow-buttercup, Ranunculus adoneus. The exposed green carpels of snow-buttercup flowers have 1–2 stomata each. Net carbon assimilation rates of flowers are negative during bud expansion, but rise to zero at maturity, and become positive during early fruit growth. Experimental removal of separate whorls of flower parts demonstrated that the showy, nectary-housing petals account for most of the respiration cost of flower presentation. Conversely, photosynthesis by female organs contributes to a flower's carbon balance. Dipteran pollinators of R. adoneus are most active in sunny mid-morning to mid-afternoon intervals. At this time of day, rates of carpel photosynthesis (Amax) meet respiratory costs of pollinator attraction in fully expanded flowers. Achenes remain photosynthetically active until dispersal, and positive net carbon assimilation rates characterize infructescences throughout fruit maturation. Photosynthetic rates of achenes are positively correlated with infructescence growth rates. We tested the causal basis of this relationship by experimentally shading developing infructescences. Mature achenes from shaded infructescences were 16–18% smaller than those from unshaded controls. Leaf photosynthetic rates did not differ between plants bearing shaded and unshaded seed heads. Since female reproductive organs are only 8% more costly in terms of caloric investment than male ones and contribute to their own carbon balance, it is plausible that the energy cost of male function equals or exceeds that of female function in this hermaphroditic species.
Similar content being viewed by others
References
Abrahamson WG, Caswell H (1982) On the comparative allocation of biomass, energy and nutrients in plants. Ecology 63:982–991
Bateman AJ (1952) Self-incompatibility systems in angiosperms. Heredity 6:285–310
Bazzaz FA, Carlson RW (1979) Photosynthetic contribution of flowers and seeds to reproductive effort of an annual colonizer. New Phytol 82:223–232
Bazzaz FA, Carlson RW, Harper JL (1979) Contribution to reproductive effort by photosynthesis of flowers and fruits. Nature 279:551–552
Biscoe PV, Gallagher JN, Littleton EJ, Monteith JL, Scott RK (1975) Barley and its environment IV. Sources of assimilate for the grain. J Appl Ecol 12:295–318
Blanke MM, Lenz F (1989) Fruit photosynthesis. Plant Cell Environ 12:31–46
Chapin FS (1989) The cost of tundra plant structures: evaluation of concepts and currencies. Am Nat 133:1–19
Charlesworth B, Charlesworth D (1978) A model for the evolution of dioecy and gynodioecy. Am Nat 112:975–997
Charlesworth B, Charlesworth D (1987) The effect of investment in attractive structures on allocation to male and female function in plants. Evolution 41:948–968
Charnov EL, Bull JJ (1986) Sex allocation, pollinator attraction and fruit dispersal in cosexual plants J Theor Biol 118:321–325
Cipollini ML, Levey DJ (1991) Why some fruits are green when they are ripe: carbon balance in fleshy fruits. Oecologia 88:371–377
Columbo B, Baccanti M, Dutko B (1987) Calculation of the heat value of solid and liquid fuels. American Laboratory, Aug. 1987, 2 pp.
Dawson TE, Ehleringer JR (1993) Gender-specific physiology, carbon isotope discrimination and habitat distribution in box elder, Acer negundo. Ecology 74:798–815
Flinn AM, Atkins CA, Pate JS (1977) Significance of photosynthetic and respiratory exchanges in the carbon economy of the developing pea fruit. Plant Physiol 60:412–418
Galen C, Stanton ML (1991) Consequences of emergence phenology for reproductive success in Ranunculus adoneus (Ranunculaceae). Am J Bot 78:978–988
Goldman DA, Willson MF (1986) Sex allocation in functionally hermaphroditic plants: a review and critique. Bot Gaz 52:157–194
Goldstein G, Sharifi MR, Kohorn LU, Lighton JRB, Shultz L, Rundel PW (1991) Photosynthesis by inflated pods of a desert shrub, Isomeris arborea. Oecologia 85:396–402
Heilmeier H, Whale DM (1987) Carbon dioxide assimilation in the flower head of Arctium. Oecologia 73:109–115
Hole CC, Scott PA (1981) The effect of fruit shading on yield in Pisum sativum L. Ann Bot 48:827–835
Jurik TW (1985) Differential costs of sexual and vegetative reproduction inwild strawberry populations. Oecologia 66:394–403
Laties GG (1978) The development and control of respiratory pathways in slices of plant storage organs. In: G. Kahl (ed) Biochemistry of wounded plant tissues. Walter de Gruyter Publ, New York, pp 421–466
Lloyd DG (1984) Gender allocations in outcrossing cosexual plants. In: Dirzo R, Sarukhan J (eds) Perspectives on Plant Population Ecology. Sinauer press, Sunderland, Massachusetts, pp 277–300
Pleasants JM, Chaplin SJ (1983) Nectar production rates of Asclepias quadrifolia: causes and consequences of individual variation. Oecologia 59:232–238
Reekie EG, Bazzaz FA (1987a) Reproductive effort in plants. 1. Carbon allocation to reproduction. Am Nat 129:876–896
Reekie EG, Bazzaz FA (1987b) Reproductive effort in plants 2. Does carbon reflect the allocation of other resources. Am Nat 129:897–906
Sambo EY, Moorby J, Milthorpe FL (1977) Photosynthesis and respiration of developing soybean pods. Aust J Plant Physiol 4:713–721
SAS Institute Inc (1985) User's guide, 1985 Ed.: Statistics. SAS Inst, Cary, NC, USA
Southwick EE (1984) Photosynthate allocation to floral nectar: a neglected energy investment. Ecology 65:1775–1779
Stanton ML, and Galen C (1989) Consequences of flower heliotropism for reproduction in an alpine buttercup (Ranunculus adoneus). Oecologia 78:477–485
Tissue DT, Nobel PS (1990) Carbon relations of flowering in a semelparous clonal desert perennial. Ecology 71:271–281
Van Voorhies WA (1992) Production of sperm reduces nematode lifespan. Nature 360:456–458
Vu JCV, Yelenosky G, Bausher MG (1985) Photosynthetic activity in the flower buds of “Valencia” Orange (Citrus sinensis [L.] Osbeck). Plant Physiol 78:420–423
Wardlaw IF (1990) The control of carbon partitioning in plants. New Phytol 116:341–381
Weiss D, Shomer-Ilan A, Halevy AH (1990) Photosynthesis in flowers of Petunia hybrida: low CO2 flow and coordinated reduction between photosynthetic systems. In: Altscheffsky M (ed) Curr Res Photosynth IV, Kluwer Acad Publ, The Netherlands, pp 417–419
Werk KS, Ehleringer JR (1983) Photosynthesis by flowers in Encelia farinosa and Encelia californica (Asteraceae). Oecologia 57:311–315
Williams K, Koch GW, Mooney HA (1985) The carbon balance of flowers of Diplacus aurantiacus. Oecologia 66:530–535
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Galen, C., Dawson, T.E. & Stanton, M.L. Carpels as leaves: meeting the carbon cost of reproduction in an alpine buttercup. Oecologia 95, 187–193 (1993). https://doi.org/10.1007/BF00323489
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00323489