Abstract
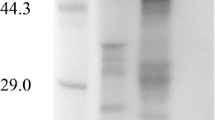
The cDNA encoding the endo-β-1,4-glucanase (carboxymethylcellulase; CMCase-I) from Aspergillus kawachii IFO 4308 was cloned. Nucleotide-sequence analysis of the cloned cDNA insert showed a 717-bp open reading frame that encoded a protein of 239 amino-acid residues. The predicted amino-acid sequence of the mature protein had considerable homology with the protein sequence of the FI-CMCase of Aspergillus aculeatus. The cDNA was introduced into Saccharomyces cerevisiae. The expressed enzyme had carboxylmethylcellulase acitivity, identified by clear zones on a CMC-agar plate after Congo Red staining.
Similar content being viewed by others
References
Béguin P (1990) Molecular biology of cellulose degradation. Annu Rev Microbiol 44:219–248
Chen CM, Gritzali M, Stafford DW (1987) Nucleotide sequence and deduced primary structure of cellobiohydrolase II from Trichoderma reesei. Bio/Technology 5:274–278
Cheng C, Tsukagoshi N, Udaka S (1990) Nucleotide sequence of the cellobiohydrolase gene from Trichoderma viride. Nucleic Acids Res 18:5559
Christensen T, Woeldike H, Boel E, Mortensen SB, Hjortshoej K, Thim L, Hansen MT (1988) High-level expression of recombinant genes in Aspergillus oryzae. Bio/Technology 6:1419–1422
Farkas V, Lísková M, Biely P (1985) Novel media for detection of microbial producers of cellulase and xylanase. FEMS Microbiol Lett 28:137–140
Gilkes NR, Henrissat B, Kilburn DG, Miller RC Jr, Warren RAJ (1991) Domains in microbial β-1,4-glycanases: sequence conservation, function, and enzyme families. Microbiol Rev 55:303–315
Hanahan D (1983) Studies of transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580
Hata Y, Kitamoto K, Gomi K, Kumagai C, Tamura G, Hara S (1991) The glucoamylase cDNA from Aspergillus oryzae: its cloning, nucleotide sequence, and expression in Saccharomyces cerevisiae. Agric Biol Chem 55:941–949
Henrissat B, Driguez H, Viet C, Schülein M (1985) Synergism of cellulases from Trichoderma reesei in the degradation of cellulose. Bio/Technology 3:722–726
Henrissat B, Claeyssens M, Tomme P, Lemesle L, Mornon J-P (1989) Cellulase families revealed by hydrophobic cluster analysis. Gene 81:83–95
Ito H, Fukuda Y, Murata K, Kimura A (1983) Transformation of intact yeast cells treated with alkali cations. J Bacteriol 153:163–168
Ito K, Ogasawara H, Sugimoto T, Ishikawa T (1992a) Purification and properties of acid stable xylanases from Aspergillus kawachii. Biosci Biotech Biochem 56:547–550
Ito K, Ikemasu T, Ishikawa T (1992b) Cloning and sequencing of the xynA gene encoding xylanase A of Aspergillus kawachii. Biosci Biotech Biochem 56:906–912
Juy M, Amit AG, Alzari PM, Poljak RJ, Claeyssens M, Béguin P, Aubert J-P (1992) Three-dimensional structure of a thermostable bacterial cellulase. Nature 357:89–91
Kamei K, Yamamura Y, Hara S, Ikenaka T (1989) Amino-acid sequence of chitinase from Streptomyces erythraeus. J Biochem 105:979–985
Knowles J, Lehtovaara P, Teeri T (1987) Cellulase families and their genes. Trends Biotechnol 5:255–261
laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Messing J (1983) New M13 vector for cloning. Methods Enzymol 101:20–78
Mikami S, Iwano K, Shiinoki S, Shimada T (1987) Purification and some properties of acid-stable α-amylase from shochu koji (Aspergillus kawachii). Agric Biol Chem 51:2495–2501
Ooi T, Shinmyo A, Okada H, Hara S, Ikenaka T, Murao S, Arai M (1990) Cloning and sequence analysis of a cDNA for cellulase (FI-CMCase) from Aspergillus aculeatus. Curr Genet 18:217–222
Penttilä M, Lehtovaara P, Nevalainen H, Bhikhabhai R, Knowles J (1986) Homology between cellulase genes of Trichoderma reesei: complete nucleotide sequence of the endoglucanase 1 gene. Gene 45:253–263
Rouvinen J, Bergfors T, Teeri T, Knowles JKC, Jones TA (1990) Three-dimensional structure of cellobiohydrase II from Trichoderma reesei. Science 249:380–386
Saloheimo M, Lehtovaara P, Penttilä M, Teeri TT, Ståhlberg J, Johansson G, Pettersson G, Claeyssens M, Tomme P, Knowles JKC (1988) EGIII, a new endoglucanase from Trichoderma reesei: the characterization of both gene and enzyme. Gene 63:11–21
Schena M, Picard D, Yamamoto KR (1991) Vectors for constutive and inducible gene expression in yeast. Methods Enzymol 194: 389–398
Shoemaker S, Schweickart V, Ladner M, Gelfand D, Kwok S, Myambo K, Innis M (1983) Molecular cloning of exo-cellobiohydrolase I derived from Trichoderma reesei strain L27. Bio/Technology 1:691–696
Sims P, James C, Broda P (1988) The identification, molecular cloning and characterisation of a gene from Phanerochaete chrysosporium that shows strong homology to the exo-cellobiohydrolase I gene from Trichoderma reesei. Gene 74:411–422
Ståhlberg J, Johansson G, Pettersson G (1991) A new model for enzymatic hydrolysis of cellulose based on the two-domain structure of cellobiohydrolase I. Bio/Technology 9:286–290
Terri T, Salovuori I, Knowles J (1983) The molecular cloning of the major cellulase gene from Trichoderma reesei. Bio/Technology 1:696–699
Turnbull IF, Rand K, Willetts NS, Hynes MJ (1989) Expression of the Escherichia coli enterotoxin subunit B gene in Aspergillus nidulans directed by the amdS promoter. Bio/Technology 7:169–174
Upshall A, Kumar AA, Bailey MC, Parker MD, Favreau MA, Lewison KP, Joseph ML, Maraganore JM, McKnight GL (1987) Secretion of active human tissue plasminogen activator from the filamentous fungus Aspergillus nidulans. Bio/Technology 5:1301–1304
Ward M, Wilson LJ, Kodama KH, Rey MW, Berka RM (1990) Improved production of chymosin in Aspergillus by expression as a glucoamylase-chymosin fusion. Bio/Technology 8:435–440
Wood TM, Bhat KM (1988) Methods for measuring cellulase activities. Methods Enzymol 160:87–112
Author information
Authors and Affiliations
Additional information
Communicated by M. Yamamoto
Rights and permissions
About this article
Cite this article
Sakamoto, S., Tamura, G., Ito, K. et al. Cloning and sequencing of cellulase cDNA from Aspergillus kawachii and its expression in Saccharomyces cerevisiae . Curr Genet 27, 435–439 (1995). https://doi.org/10.1007/BF00311212
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00311212