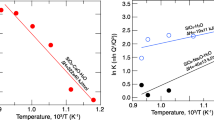
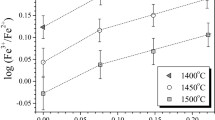
Abstract
Ultrasonic longitudinal acoustic velocities in oxidized silicate liquids indicate that the pressure derivative of the partial-molar volume of Fe2O3 is the same in iron-rich alkali-, alkaline earth- and natural silicate melt compositions at 1 bar. The dV/dP for multicomponent silicate liquids can be expressed as a linear combination of partial-molar constants plus a positive excess term for Na2O−Al2O3 mixing. Partial-molar properties for FeO and Fe2O3 components allow extension of the empirical expression of Sack et al. (1980) to permit the calculation of Fe-redox equilibrium in a natural silicate liquid as a function of composition, temperature, fo2 and pressure; a more formal thermodynamic expression is presented in the Appendix. The predicted equilibrium fo2 of natural silicate melts, of fixed oxygen content, closely parallels that defined by the metastable assemblage fayalite+magnetite+β-quartz (FMQ), in pressure-temperature space. A silicate melt initially equilibrated at 3 GPa and FMQ, will remain within approximately 0.5 log10 units of FMQ during its closed-system ascent. Thus, for magmas closed to oxygen, iron-redox equilibrium in crystal-poor pristine glassy lavas represents an excellent probe of the relative oxidation state of their source regions.
Similar content being viewed by others
References
Baidov VV, Kunin LL (1968) Speed of ultrasound and compressibility of molten silica. Sov Phys — Dokl 13:64–65
Becker GE (1986) Velocity of sound. In: Weast RC, Astle MI, Beyer WH (eds) CRC handbook of chemistry and physics, 66th. edn. CRC Press, Boca Raton, Florida, p E-43
Berman RG (1988) Internally-consistent thermodynamic data for minerals in the system Na2O−K2O−CaO−MgO−FeO−Fe2O3−Al2O3−SiO2−TiO2−H2O−CO2. J Petrol 29:445–522
Birch F (1966) Compressibility; elastic constants. In: Clark SPJr (ed) Handbook of physical constants-revised edition. Geol Soc Am Mem 97, pp 97–107
Bockris JO'M, Kojonen E (1960) The compressibilities of certain molten silicates and borates. J Am Chem Soc 82:4493–4497
Bowker JC, Lupis CHP, Flinn PA (1981) Structural studies of slags by Mössbauer spectroscopy. Can Metall Q 20:69–78
Carmichael RS (1984) Handbook of physical properties of rocks Volume III. CRC Press, Boca Raton, Florida
Carmichael ISE, Ghiorso MS (1986) Oxidation-reduction relations in basic magma: a case for homogenious equilibria. Earth Planet Sci Lett 78:200–210
Dingwell DB, Brearley M (1988) Melt densities in the CaO−FeO−Fe2O3−SiO2 system and the compositional dependence of the partial molar volume of ferric iron in silicate melts. Geochim Cosmochim Acta 52:2815–2825
Dingwell DB, Brearley M, Dickinson JEJr (1988) Melt densities in the Na2O−FeO−Fe2O3−SiO2 system and the partial molar volume of tetrahedrally-coordinated ferric iron in silicate melts. Geochim Cosmochim Acta 52:2467–2475
Dyer MD, Naney MT, Swanson SE (1987) Effects of quench methods on Fe3+/Fe2+ ratios: a Mössbauer and wet-chemical study. Am Mineral 72:792–800
Fudali RF (1965) Oxygen fugacities of basaltic and andesitic magmas. Geochim Cosmochim Acta 29:1063–1075
Greig JW (1927a) Immiscibility in silicate melts: part I. Am J Sci 5:1–44
Greig JW (1927b) Immiscibility in silicate melts: part II. Am J Sci 5:133–154
Holmes RD, O'Neill HStC, Arculus RJ (1986) Standard Gibbs free energy of formation for Cu2O, NiO, CoO, and FexO: high resolution electrochemical measurements using zirconia solid electrolytes from 900–1,400 K. Geochim Cosmochim Acta 50:2439–2452
Iwamoto N, Tsumawaki Y, Nakagawa H, Yoshimura T, Wakabayashi N (1978) Investigation of calcium-iron-silicate glasses by the Mössbauer method. J of Non-Cryst Solids 29:347–356
Kennedy GC (1948) Equilibrium between volatiles and iron oxides in igneous rocks. Am J Sci 246:529–549
Kilinc A, Carmichael ISE, Rivers ML, Sack RO (1983) The ferricferrous ratio of natural silicate liquids equilibrated in air. Contrib Mineral Petrol 83:136–140
Kress VC (1990) Experiments in Silicate Liquids: Redox State and Sound Speeds. PhD Thesis, University of California, Berkeley
Kress VC, Carmichael ISE (1988) Stoichiometry of the iron oxidation reaction in silicate melts. Am Mineral 73:1267–1274
Kress VC, Carmichael ISE (1989) The lime-iron-silicate melt system: redox and volume systematics. Geochim Cosmochim Acta 53:2883–2892
Kress VC, Williams Q, Carmichael ISE (1988) Ultrasonic investigation of melts in the system Na2O−Al2O3−SiO2. Geochim Cosmochim Acta 52:283–293
Kress VC, Williams Q, Carmichael ISE (1989) When is a silicate melt not a liquid. Geochim Cosmochim Acta 53:1687–1692
Lange R, Carmichael ISE (1987) Densities of Na2O−K2O−CaO−MgO−FeO−Fe2O3−Al2O3−TiO2−SiO2 liquids: new measurements and derived partial-molar properties. Geochim Cosmochim Acta 51:2931–2946
Lange RA, Carmichael ISE (1989) Ferric-ferrous equilibria in Na2O−FeO−Fe2O3−SiO2 melts: effects of analytical techniques on derived partial molar volumes. Geochim Cosmochim Acta 53:2195–2204
Levy RA, Lupis CHP, Flinn PA (1976) Mössbauer analysis of the valence and coordination of iron cations in SiO2−Na2O−CaO glasses. Phys Chem Glasses 17:94–103
Lindsley DH, Speidel DH, Nafziger RH (1968) P-T-fo2 relations for the system Fe−O−SiO2. Am J Sci 226:342–360
Manghnani MH, Sato H, Rai CS (1986) Ultrasonic velocity and attenuation measurements on basalt melts to 1,500° C: role of composition and structure in the viscoelastic properties. J Geophys Res 91:9333–9342
Mo X, Carmichael ISE, Rivers M, Stebbins J (1982) The partial molar volume of Fe2O3 in multicomponent silicate liquids and the pressure dependence of oxygen fugacity in magmas. Mineral Mag 45:237–245
Murase T, McBirney AR (1973) Properties of some common igneous rocks and their melts at high temperatures. Geol Soc Am Bull 84:3563–3592
Navrotsky A, Geisinger KL, McMillan P, Gibbs GV (1985) The tetrahedral framework in glasses and melts: inference from molecular orbital calculations and implications for structure, thermodynamics and physical properties. Phys Chem Miner 11:284–298
Notis MR, Spriggs RM, Hahn WCJr (1971) Elastic moduli of pressure-sintered nickel oxide. J Geophys Res 76:7052–7061
O'Horo MP, Levy RA (1978) Effect of melt atmosphere on the magnetic properties of a [(SiO2)45(CaO)55]65[Fe2O3]35 glass. J Appl Phys 49:1635–1637
Pargamin L, Lupis CHP, Flinn PA (1972) Mössbauer analysis of the distribution of iron cations in silicate slags. Metall Trans 3:2093–2105
Riebling EF (1966) Structure of sodium aluminosilicate melts containing at least 50 mol% SiO2 at 1,500° C. J Chem Phys 44:2857–2865
Rigden SM, Ahrens TJ, Stolper EM (1984) Density of liquid silicates at high pressures. Science 226:1071–1074
Rigden SM, Ahrens TJ, Stolper EM (1988) Shock compression of molten silicate: results for a model basaltic composition. J Geophys Res 93:367–382
Rigden SM, Ahrens TJ, Stolper EM (1989) High pressure equation of state of molten anorthite and diopside. J Geophys Res 94:9508–9522
Rivers ML, Carmichael ISE (1987) Ultrasonic studies of silicate melts. J Geophys Res 92:9247–9270
Sack RO, Carmichael ISE, Rivers M, Ghiorso MS (1980) Ferricferrous equilibria in natural silicate liquids at 1 bar. Contrib Mineral Petrol 75:369–376
Sato M (1978) Oxygen fugacity of basaltic magmas and the role of gas-forming elements. Geophys Res Lett 5:447–449
Skinner BJ (1966) Thermal expansion. In: Clark SPJr (ed) Handbook of physical constants-revised edition. Geol Soc Am Mem, 97, pp 75–96
Sokolov LN, Baidov VV, Kunin LL, Dymov VV (1971) Surface and volume characteristics of the calcium oxide-alumina-silica system. Sb Tr Tsentr Nauchno-Issled Inst Chem Metall 74:53–61
Stebbins JF, Carmichael ISE, Moret LK (1984) Heat capacities and entropies of silicate liquids and glasses. Contrib Mineral Petrol 86:131–148
Thornber CR, Roeder PL, Foster JR (1980) The effect of composition on the ferric-ferrous ratio in basaltic liquids at atmospheric pressure. Geochim Cosmochim Acta 44:525–532
Tomlinson JW, Henes MSR, Bockris JO'M (1958) The structure of liquid silicates-part 2: molar volumes and expansivities. Trans Faraday Soc 54:1822–1833
Whittaker EJW, Muntas R (1970) Ionic radii for use in geochemistry. Geochim Cosmochim Acta 34:945–956
Wilson AD (1960) The micro-determination of ferrous iron in silicate minerals by a volumetric and a colorimetric method. Analyst 85:823–827
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kress, V.C., Carmichael, I.S.E. The compressibility of silicate liquids containing Fe2O3 and the effect of composition, temperature, oxygen fugacity and pressure on their redox states. Contr. Mineral. and Petrol. 108, 82–92 (1991). https://doi.org/10.1007/BF00307328
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00307328