Abstract
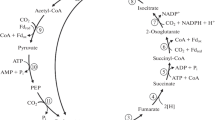
The oxidation of organic compounds with elemental sulfur or thiosulfate as electron acceptor was studied in the anaerobic hyperthermophilic archaea Thermoproteus tenax and Pyrobaculum islandicum. T. tenax was grown on either glucose or casamino acids and sulfur; P. islandicum on peptone and either elemental sulfur or thiosulfate as electron acceptor. During exponential growth only CO2 and H2S rather than acetate, alanine, lactate, and succinate were detected as fermentation products of both organisms; the ratio of CO2/H2S formed was 1:2 with elemental sulfur and 1:1 with thiosulfate as electron acceptor. Cell extracts of T. tenax and P. islandicum contained all enzymes of the citric acid cycle in catabolic activities: citrate synthase, aconitase, isocitrate dehydrogenase (NADP+-reducing), oxoglutarate: benzylviologen oxidoreductase, succinyl-CoA synthetase, succinate dehydrogenase, fumarase and malate dehydrogenase (NAD+-reducing). Carbon monoxide dehydrogenase activity was not detected. We conclude that in T. tenax and P. islandicum organic compounds are completely oxidized to CO2 with sulfur or thiosulfate as electron acceptor and that acetyl-CoA oxidation to CO2 proceeds via the citric acid cycle.
Similar content being viewed by others
References
Anfinsen CB (1955) Aconitase from pig heart muscle. In: Colowick SP, Kaplan NO (eds) Methods in enzymology, vol 1 Academic Press, New York, pp 695–698
Beh M, Strauss G, Huber R, Stetter K-O, Fuchs G (1993) Enzymes of the reductive citric acid cycle in the autotrophic eubacterium Aquifex pyrophilus and in the archaebacterium Thermoproteus neutrophilius. Arch Microbiol 160:306–311
Bergmeyer HU, Gawehn K, Graßl M (1974) Enzyme als biologische Reagentien. In: Bergmeyer HU (ed) Methoden der enzymatischen Analyse, vol 1. Verlag Chemie, Weinheim, pp 454–558
Bernt E, Bergmeyer HU (1974) Isocitrate-dehydrogenase. In: Bergmeyer HU (ed) Methoden der enzymatischen Analyse, vol 1. Verlag Chemie, Weinheim, pp 664–667
Beutler HO (1985) Succinate. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 7. Verlag Chemie, Weinheim, pp 25–33
Bock A-K, Prieger-Kraft A, Schönheit P (1984) Pyruvate—a novel substrate for growth and methane formation in Methanosarcina barkeri. Arch Microbiol 161:33–46
Bode C, Goebell H, Stähler E (1968) Zur Eliminierung von Trübungsfehlern bei der Eiweißbestimmung mit der Biuretmethode. Z Klin Chem Klin Biochem 5:418–422
Bonch-Osmolovskaya EA, Miroshnichenko ML, Kostrikina NA, Chernych NA, Zavarzin GA (1990a) Thermoproteus uzoniensis sp nov, a new extremely thermophilic archaebacterium from Kamchatka continental hot springs. Arch Microbiol 154:556–559
Bonch-Osmolovakaya EA, Sokolova TG, Kostrikina NA, Zavarzin GA (1990b) Desulfurella acetivorans gen nov and sp nov—a new thermophilic sulfur-reducing eubacterium. Arch Microbiol 153:151–155
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brandis-Heep A, Gebhard NA, Thauer RK, Widdel F, Pfenning N (1983) Anaerobic acetate oxidation to CO2 by Desulfurobacter postgatei. Arch Microbiol 136:222–229
Cline JD (1969) Spectrometric determination of hydrogen sulfide in natural waters. Limnol Oceanogr 14:454–458
Danson MJ (1993) Central metabolism of the archaea. New Compr Biochem 26:1–24
Dorn M, Andreesen JR, Gottschalk G (1978) Fermentation of fumarate and l-malate by Clostridium formicoaceticum. J Bacteriol 133:26–32
Fischer F, Zillig W, Stetter KO, Schreiber G (1983) Chemolithoautotrophic metabolism of anaerobic extremely thermophilic archaeabacteria. Nature 301:511–313
Gebhardt NA, Thauer RK, Linder D, Kaulfers P-M, Pfennig N (1985) Mechanism of acetate oxidation to CO2 with elemental sulfur in Desulfuromonas acetoxidans. Arch Microbiol 141: 392–398
Graßl M, Supp M (1985) Alanine: determination with alanineaminotransferase and lactate dehydrogenase. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 8. Verlag Chemie, Weinheim, pp 345–349
Hensel R, Laumann S, Lang J, Heumann H, Lottspeich F (1987) Characterization of two d-glyceraldehyde-3-phosphate dehydrogenases from the extremely thermophilic archaebacterium Thermoproteus tenax. Eur J Biochem 170:325–333
Huber R, Kristjansson JK, Stetter KO (1987) Pyrobaculum gen nov, a new genus of neutrophilic, rod-shaped archaebacteria from continental solfataras growing optimally at 100°C. Arch Microbiol 149:95–101
Kandler O (1993) Archaea (Archaebacteria). Prog Botany 54:1–23
Kerscher L, Osterhelt D (1982) Pyruvate: ferredoxin oxidoreductase — new finding on an ancient enzyme. Trends Biochem Sci 7:371–374
Miroshnichenko ML, Gongadze GA, Lysenko AM, Bonch-Osmolovskaya EA (1994) Desulfurella multipotens sp nov, a new sulfur-respiring thermophilic eubacterium from Raoul Island (Kermadec archipelago, New Zealand) Arch Microbiol 161: 88–93
Möller-Zinkhan D, Thauer RK (1990) Anaerobic lactate oxidation to 3 CO2 by Archaeoglobus fulgidus via the carbon monoxide dehydrogenase pathway: demonstration of the acetyl-CoA carbon-carbon cleavage reaction in cell extracts. Arch Microbiol 153:215–218
Pfennig N, Biebl H (1976) Desulfuromonas acetoxidans gen nov and sp nov, a new anaerobic, sulfur-reducing, acetate-oxidizing bacterium. Arch Microbiol 110:3–12
Schäfer T, Schönheit P (1991) Pyruvate metabolism of the hyperthermophilic archaebacterium Pyrococcus furiosus. Acetate formation from acetyl-CoA and ATP synthesis are catalyzed by an acetyl-CoA synthetase (ADP-forming) Arch Microbiol 155: 366–377
Schäfer T, Schönheit P (1992) Maltose fermentation to acetate, CO2 and H2 in the anaerobic hyperthermophilic archaeon Pyrococcus furiosus: evidence for the operation of a novel sugar fermentation pathway. Arch Microbiol 158:188–202
Schäfer S, Barkowski C, Fuchs G (1986) Carbon assimilation by the autotrophic thermophilic archaebacterium Thermoproteus neutrophilus. Arch Microbiol 146:301–308
Schäfer T, Selig M, Schönheit P (1993) Acetyl-CoA synthetase (ADP-forming) in archaea, a novel enzyme involved in acetate formation and ATP synthesis. Arch Microbiol 159:72–83
Schauder R, Kröger A (1993) Bacterial sulphur respiration. Arch Microbiol 159:491–497
Schauder R, Eikmanns B, Thauer RK, Widdel F, Fuchs G (1986) Acetate oxidation to CO2 in anaerobic bacteria via a novel pathway not involving reactions of the citric acid cycle. Arch Microbiol 145:162–172
Schauder R, Widdel F, Fuchs G (1987) Carbon assimilation pathway in sulfate-reducing bacteria. 2. Enzymes of the reductive citric acid cycle in the autotrophic Desulfobacter hydrogenophilus. Arch Microbiol 148:218–255
Schmitz RA, Bonch-Osmolovskaya EA, Thauer RK (1990) Different mechanisms of acetate activation in Desulfurella acetivorans and Desulfuromonas acetoxidans. Arch Microbiol 154: 274–279
Schönheit P, Wäscher C, Thauer RK (1978) A rapid procedure for the purification of ferredoxin from Clostridium pasteurianum using polyethyleneimine. FEBS Lett 89:219–222
Shiba H, Kawasumi T, Igarashi Y, Kodoma T, Minoda Y (1985) The Co2 assimilation via the reductive tricarboxylic acid cycle in an obligately autotrophic aerobic hydrogen-oxidizing bacterium, Hydrogenobacter thermophilus. Arch Microbiol 142: 198–203
Siebers B, Hensel R (1993) Glucose catabolism of the hyperthermophilic archaeum Thermoproteus tenax. FEMS Microbiol Lett 111:1–8
Stetter KO (1993) Life at the upper temperature border. In: Tran Thanh Van J, Tran Thanh Van K, Mounolou JC, Schneider J, McKay C. Colloque Interdisciplinare du Comité de la Recherche Scientifique, Frontiers of life, C55. Edition Frontiers, Gif-sur-Yvette, pp 195–219
Stetter KO, Lauerer G, Thomm M, Neuner A (1987) Isolation of extremely thermophilic sulfate-reducers: evidence for a novel branch of archaebacteria. Science 236:822–824
Stetter KO, Fiala G, Huber G, Huber R, Segerer A (1990) Hyperthermophilic microorganisms. FEMS Microbiol Rev 75:117–124
Thauer RK (1988) Citric-acid cycle, 50 years on. Modifications and an alternative pathway in anaerobic bacteria. Eur J Biochem 179:497–508
Thauer RK, Möller-Zinkhan D, Spormann A (1989) Biochemistry of acetate catabolism in anaerobic chemotrophic bacteria. Annu Rev Microbiol 43:43–67
Thurl S, Buhrow I, Schäfer W (1985) Quinones from archaebacteria. I. New types of menaquinones from the thermophilic archaebacterium Thermoproteus tenax. Biol Chem Hoppe Seyler 366:1079–1083
Tindall BJ (1989) Fully saturated menaquinones in the archaebacterium Pyrobaculum islandicum. FEMS Microbiol Let 60: 251–254
Wächtershäuser G (1990) Evolution of the first metabolic cycles. Proc Natl Acad Sci USA 87:200–204
Weissbach A, Hurwitz J (1959) The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli. Br J Biol Chem 234:705–709
Widdel F, Pfennig N (1981) Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. I. Isolation of new sulfate-reducing bacteria enriched with acetate from saline environments. Description of Desulfobacter postgatei gen nov, sp nov. Arch Microbiol 129:395–400
Woese CR, Kandler O, Wheelis ML (1990) Towards a natural system of organisms: proposal for the domains archaea, bacteria, and eucarya. Proc Natl Acad Sci USA 87:4576–4579
Zillig W, Stetter KO, Schäfer W, Janekovic D, Wunderl S, Holz I, Palm P (1981) Thermoproteales: a novel type of extremely thermoacidophilic anaerobic archaebacteria isolated from Islandic solfataras. Zentralbl Bakteriol Hyg C2:205–227
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Selig, M., Schönheit, P. Oxidation of organic compounds to CO2 with sulfur or thiosulfate as electron acceptor in the anaerobic hyperthermophilic archaea Thermoproteus tenax and Pyrobaculum islandicum proceeds via the citric acid cycle. Arch. Microbiol. 162, 286–294 (1994). https://doi.org/10.1007/BF00301853
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00301853