Abstract
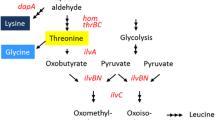
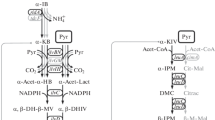
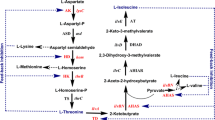
Overproduction of isoleucine, an essential amino acid, was achieved by amplification of the gene encoding threonine dehydratase, the first enzyme in the threonine to isoleucine pathway, in a Corynebacterium lactofermentum threonine producer. Threonine overproduction was previously achieved with C. lactofermentum ATCC 21799, a lysine-hyperproducing strain, by introduction of plasmid pGC42 containing the Corynebacterium hom dr and thrB genes (encoding homoserine dehydrogenase and homoserine kinase respectively) under separate promoters. The pGC42 derivative, pGC77, also contains ilvA, which encodes threonine dehydratase. In a shake-flask fermentation, strain 21799(pGC77) produced 15 g/l isoleucine, along with small amounts of lysine and glycine. A molar carbon balance indicates that most of the carbon previously converted to threonine, lysine, glycine and isoleucine was incorporated into isoleucine by the new strain. Thus, in our system, simple overexpression of wild-type ilvA sufficed to overcome the effects of feedback inhibition of threonine dehydratase by the end-product, isoleucine.
Similar content being viewed by others
References
Bell SC, Turner JM (1976) Bacterial catabolism of threonine. Threonine degradation initiated by L-threonine-NAD+ oxidoreductase. Biochem J 156:449–458
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principles of protein-dye binding. Anal Biochem 72:248–254
Colón GE, Jetten MSM, Nguyen TT, Gubler M, Follettie MT, Sinskey AJ, Stephanopoulos G (1995) Effect of inducible thrB expression on amino acid production in Corynebacterium lactofermentum 21799. Appl Environ Microbiol 61:74–78
Cordes C, Möckel B, Eggeling L, Sahm H (1992) Cloning, organization and functional analysis of ilvA, ilvB and ilvC genes from Corynebacterium glutamicum. Gene 112:113–116
Decedue CJ, Hofler JG, Burns RO (1975) Threonine deaminase from Salmonella typhymurium. Relationship between regulatory sites. J Biol Chem 250:1563–1570
Eggeling I, Cordes C, Eggeling L, Sahm H (1987) Regulation of acetohydroxy acid synthase in Corynebacterium glutamicum during fermentation of α-ketobutyrate to L-isoleucine. Appl Microbiol Biotechnol 25:346–351
Follettie MT (1989) Development of recombinant DNA technology for Corynebacterium glutamicum:isolation and characterization of amino acid biosynthetic genes. PhD thesis, Massachusetts Institute of Technology, Cambridge, Mass
Follettie MT, Sinskey AJ (1986) Molecular cloning and nucleotide sequence of the Corynebacterium glutamicum pheA gene. J Bacteriol 167:695–702
Follettie MT, Archer J, Peoples OP, Sinskey AJ (1991) Metabolic engineering of Corynebacterium. In: Heslot H, Davies J, Florent J, Bobichon L, Durand G, Penasse L (eds) Proceedings of the Sixth International Symposium on Genetics of Industrial Microorganisms (GIM 90), vol 1, Strasbourg, France, pp 315–325
Follettie MT, Peoples OP, Agoropoulou C, Sinskey AJ (1993) Gene structure and expression of the Corynebacterium flavum N13 ask-asd operon. J Bacteriol 175:4096–4103
Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580
Hatfield GW, Umbarger HE (1970) Threonine deaminase from Bacillus subtilis. II. The steady state kinetic properties. J Biol Chem 245:1742–1747
Jetten MSM, Sinskey AJ (1993) Characterization of phosphoenolpyruvate carboxykinase from Corynebacterium glutamicum. FEMS Microbiol Lett 111:183–188
Jetten MSM, Gubler ME, McCormick MM, Colón GE, Follettie MT, Sinskey AJ (1993) Molecular organization and regulation of the biosynthetic pathway for aspartate-derived amino acids in Corynebacterium glutamicum. In: Baltz RH, Hegeman GD, Skalriud PL (eds) Industrial microorganisms: basic and applied molecular genetics, vol 13. American Society of Microbiology, Washington, pp 97–104
Kase H, Nakayama K (1977) L-Isoleucine production by analog-resistant mutants derived from threonine-producing strain of Corynebacterium glutamicum. Agric Biol Chem 41:109–116
Keilhauer C, Eggeling L, Sahm H (1993) Isoleucine synthesis in Corynebacterium glutamicum: molecular analysis of the ilvB-ilvN-ilvC operon. J Bacteriol 175:5595–5603
Kinoshita S (1985) Glutamic acid bacteria. In: Demain AL, Solomon NA (eds) Biology of industrial microorganisms. Benjamin/Cummings London, pp 115–142
Kiss R (1991) Metabolic activity control of the L-lysine fermentation by restrained growth fed-batch strategies. PhD thesis, Masschusetts Institute of Technology, Cambridge, Mass
Lennox ES (1995) Transduction of linked genetic characters of the host by bacteriophage P1. Virology 1:190–206
Liebl W (1991) The genus Corynebacterium-non-medical. In: Balows A, Truper HG, Dworkin M, Harder W, Schleifer K-H (eds) The procaryotes. Springer, Berlin Heidelberg New York, pp 1157–1171
Liebl W, Ehrmann M, Ludwig W, Schleifer KH (1991) Transfer of Brevibacterium divaricatum DSM 20297T, “Brevibacterium flavum” DSM 20411, “Brevibacterium lactofermentum” DSM 20412 and DSM 1412, and Corynebacterium lilium DSM 20137T to Corynebacterium glutamicum and their distinction by rRNA gene restriction patterns. Int J Syst Bacteriol 41:255–260
Miyajima R, Shiio I (1972) Regulation of aspartate family amino acid biosynthesis in Brevibacterium flavum. Effects of isoleucine and valine on threonine dehydratase activity and its formation. J Biochem (Tokyo) 71:951–960
Miyajima R, Otsuka S-I, Shiio I (1968) Regulation of aspartate family amino acid biosynthesis in Brevibacterium flavum. I. Inhibition by amino acids of the enzymes in threonine biosynthesis. J Biochem (Tokyo) 63:139–148
Möckel B, Eggeling L, Sahm H (1992) Functional and structural analyses of threonine dehydratase from Corynebacterium glutamicum. J Bacteriol 174:8065–8072
Osten C von der, Gioannetti C, Sinskey AJ (1989) Design of a defined medium for growth of Corynebacterium glutamicum in which citrate facilitates iron uptake. Biotechnol Lett 11:11–16
Nakamori S, Ishida M, Tagaki H, Ito K, Miwa K, Sano K (1987) Improved L-threonine production by the amplification of the gene encoding homoserine dehydrogenase in Brevibacterium lactofermentum. Agric Biol Chem 51:87–91
Reinscheid DJ, Kronemeyer W, Eggeling L, Eikmanns BJ, Sahm H (1994) Stable expression of hom-1-thrB in Corynebacterium glutamicum and its effects on the carbon flux to threonine and related amino acids. Appl Environ Microbiol 60:126–132
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. A laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, New York
Scheer E, Cordes C, Eggeling L, Sahm H (1987) Regulation of acetohydroxy acid synthase in Corynebacterium glutamicum during isoleucine formation from α-hydroxybutyric acid. Arch Microbiol 149:173–174
Tsuchida T, Momose H (1975) Genetic changes of regulatory mechanisms occured in leucine and valine producing mutants derived from Brevibacterium lactofermentum 2256. Agric Biol Chem 39:2193–2198
Umbarger HE (1956) Evidence for a negative-feedback mechanism. Science 123:848
Vieira J, Messing J (1982) The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19:259–268
Westerfeld WW (1945) A colorimetric determination of blood acetoin. J Biol Chem 161:495–502
Yoshihama M, Higashiro K, Rao EA, Akedo M, Shanabruch WG, Follettie MT, Walker GC, Sinskey AJ (1985) Cloning vector system for Corynebacterium glutamicum. J Bacteriol 162:591–597
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Colón, G.E., Nguyen, T.T., Jetten, M.S.M. et al. Production of isoleucine by overexpression of ilvA in a Corynebacterium lactofermentum threonine producer. Appl Microbiol Biotechnol 43, 482–488 (1995). https://doi.org/10.1007/BF00218453
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00218453