Summary
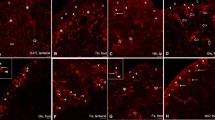
The general structure, ultrastructure and innervation of the swimbladder of the smooth toadfish, Tetractenos glaber, were examined with light-microscopic, fluorescence-histochemical, and transmission electron-microscopic techniques. The structure of the swimbladder is similar to that of other euphysoclists. Fluorescence histochemistry showed adrenergic fibres in both the secretory and resorptive areas of the swimbladder. Transmission electron microscopy revealed two morphologically distinct axon profiles type-I profiles containing many small, flattened vesicles; type-II profiles containing both large, granular vesicles and rounded, small clear vesicles in varying proportions.
The gas-gland cells and surrounding muscularis mucosae are innervated by both type-I and type-II fibres. Type-I fibres also innervate pre-rete arteries. The rete- and gas-gland capillaries do not appear to be innervated. Arteries running to the resorptive area are innervated by type-I fibres. Both type-I and type-II profiles make contact with the muscularis mucosae in the resorptive area. Only type-I fibres innervate the radial dilator muscle in the oval sphincter region, whereas only type II fibres innervate the circular muscle of the oval sphincter.
Type-I fibres took up α-methyl-noradrenaline, and could not be found after pre-treatment with 6-hydroxydopamine. They are, therefore, assumed to be adrenergic. Type-II fibres were tentatively identified, by exclusion, as cholinergic.
Similar content being viewed by others
References
Augustinsson KB, Fänge R (1951) Innervation and acetylcholine splitting activity in the airbladder of fishes. Acta Physiol Scand 22:224–230.
Bennett HS, Wyrick AD, Lee SW, McNeil JH (1976) Science and art in preparing tissues embedded in plastic for light microscopy with special reference to glycol methacrylate, glass knives and simple stains. Stain Technol 51:71–97.
Berger PJ, Gibbins IL, Hards DK, Crosby LJ (1982) The distribution and ultrastructure of sensory elements in the baroreceptor region of the truncus arteriosus of the lizard, Trachydosaurus rugosus. Cell Tissue Res 226:389–406.
Burnstock G, Costa M (1975) Adrenergic neurons. Chapman and Hall, London.
Burnstock G, Iwayama T (1971) Fine structural identification of autonomic nerves and their relation to smooth muscle. Prog Brain Res 34:389–404.
Copeland E (1960) Secretory epithelium of the swim bladder in Fundulus. Biol Bull Woods Hole 119:311–312.
Deineka D (1905) Zur Frage über den Bau der Schwimmblase. Z Wiss Zool 78:149–164.
Fänge R (1953) The mechanism of gas transport in the euphysoclist swimbladder. Acta Physiol Scand 30 Suppl 110:1–133.
Fänge R (1966) Physiology of the swimbladder. Physiol Rev 46:299–322.
Fänge R (1983) Gas exchange in fish swim bladder. Physiol Biochem Pharmacol 97:111–158.
Fänge R, Holmgren S (1982) Choline acetyltransferase activity in the fish swimbladder. J Comp Physiol 146:57–61.
Fänge R, Holmgren S, Nilsson S (1976) Autonomic nerve control of the swimbladder of the goldskinny wrasse, Ctenolabrus repestris. Acta Physiol Scand 97:292–303.
Fahlen G, Falck B, Rosengren E (1965) Monoamines in the swimbladder of Gadus callarias and Salmo irideus. Acta Physiol Scand 64:119–126.
Furness JB, Costa M (1975) The use of glyoxylic acid for the fluoresence histochemical demonstration of peripheral stores of noradrenaline and 5-hydroxytryptamine in whole mounts. Histochemistry 41:335–352.
Gibbins I (1982) Lack of correlation between ultrastructural and pharmacological types of non-adrenergic autonomic nerves. Cell Tissue Res 221:551–581.
Gibbins IL, Haller CJ (1979) Ultrastructural identification of non-adrenergic non-cholinergic nerves in the rat anoccygeus muscle. Cell Tissue Res 200:257–271.
Hards DK (1983) Stain removal from methacrylate sections. Med Lab Sci 40:393–394.
Hardy GS (1983) Revision of Australian species of Torquigener Whitley (Tetraodontiformes: Tetraodontidae), and two new generic names for Australian puffer fishes. J R Soc NZ 13:1–48.
Jasiński A, Kilarski W (1969) On the fine structure of the gas gland in some fishes. Z Zellforsch 102:333–356.
McLean JR, Nilsson S (1981) A histochemical study of the gas gland innervation in the Atlantic cod, Cadus morhua. Acta Zool 62(3):187–194.
Morris S, Albright J (1975) The ultrastructure of the swimbladder of the toadfish, Opsanus tau L. Cell Tissue Res 164:85–104.
Nilsson S (1970) Autonomic innervation of the smooth muscle sphincter in the swimbladder of a fish (Gadus morhua). Acta Physiol Scand 80(4):36A-37A.
Nilsson S (1971) Adrenergic innervation and drug responses of the oval sphincter in the swimbladder of the cod (Gadus morhua). Acta Physiol Scand 83:446–453.
Nilsson S (1983) Autonomic nerve function in the vertebrates. Springer, Berlin Heidelberg New York.
Nilsson S, Fänge R (1967) Adrenergic receptors in the swimbladder and gut of a teleostean fish (Anguilla anguilla). Comp Biochem Physiol 23:661–664.
Ross LG (1978) The innervation of the resorptive structures in the swimbladder of a physoclist fish, Polachius virens (L). Comp Biochem Physiol 61C:385–388.
Saupe M (1940) Anatomic und Histologie der Schwimmblase des Flußbarsches (Perca fluviatilis) mit besonderer Berücksichtigung des Ovals. Z Zellforsch 30:1–34.
Steen JB (1970) The swimbladder as a hydrostatic organ. In: Hoar WS, Randall DJ (eds) Fish physiology. Academic Press, New York London, pp 414–440.
Tranzer JP, Richards JG (1976) Ultrastructural cytochemistry of biogenic amines in nervous tissue: methodologic improvements. J Histochem Cytochem 24:1178–1193.
Wilson AJ, Furness JB, Costa M (1981) The fine structure of the submucous plexus of the guinea-pig ileum. II. Description and analysis of vesiculated nerve profiles. J Neurocytol 10:785–804.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Green, S.L. Ultrastructure and innervation of the swimbladder of Tetractenos glaber (Tetraodontidae). Cell Tissue Res. 237, 277–284 (1984). https://doi.org/10.1007/BF00217146
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00217146