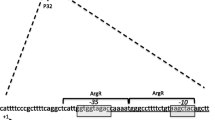
Abstract
The phytopathogenic, gram-negative bacterium Pseudomonas syringae pv. syringae 61 contains three isozymes of catalase (EC 1.11.1.6), which have been proposed to play a role in the bacterium's responses to various environmental stresses. To study the role of individual isozymes, the gene coding for the catalytic subunit of one catalase isozyme was cloned from a cosmid library hosted in Escherichia coli DH5α by using a designed catalase-specific DNA probe for the screening. One out of four clones with a catalase-positive genotype was subcloned and a pUC19-based 2.7 × 103-base (2.7-kb) insert subclone, pMK3E5, was used to transform catalase-deficient E. coli strain UM255 (HPI−, HPII−). The transformants contained a single isozyme of catalase that had electrophoretic and enzymic properties similar to catalase isozyme CatF from P. syringae pv. syringae 61. Analysis of the sequenced 2.7-kb insert DNA revealed six putative open-reading frames (ORF). The 1542-base-pair DNA sequence of ORF2, called catF, encodes a peptide of 513 amino acid residues with a calculated molecular mass of 66.6 kDa. The amino acid sequence deduced from catF had homology to the primary structure of true catalases from mammals, plants, yeasts and bacteria. The activity of the recombinant catalase was inhibited by 3-amino-1,2,4-triazole and azide and stimulated by chloramphenicol. The N terminus contained a signal sequence of 26 amino acids necessary for secretion into the periplasm, a so-far unique property of Pseudomonas catalases.
Similar content being viewed by others
References
Anderson AJ, Guerra D (1985) Responses of bean to root colonization with Pseudomonas putida in a hydroponic system. Phytopathology 75:992–995
Bishai WR, Smith HO, Barcak GJ (1994) A peroxide/ascorbate-inducible catalase from Haemophilus influenzae is homologous to the Escherichia coli katE gene product. J Bacteriol 176:2914–2921
Bol DK, Yasbin RE (1991) The isolation, cloning and identification of a vegetative catalase gene from Bacillus subtilis. Gene 109:31–37
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brown S, Anderson A, Ohman DE, Hassett DJ (1994) Cloning of the catalase gene (katE) and characterization of the purified enzyme in Pseudomonas aeruginosa: localization of katE near sodA and sodB on the genomic map. In: General meeting of the American Society for Microbiology Abstracts. American Society for Microbiology, Washington, DC, p 34
Claiborne A, Fridovich I (1979) Purification of the o-diasidine peroxidase from Escherichia coli B. J Biol Chem 254:4245–4252
Del Sal G, Manfioletti G, Schneider C (1989) The CTAB-DNA precipitation method: a common mini-scale preparation of template DNA from phagmids, phages, or plasmids suitable for sequencing. Biotechniques 7:514–519
Devereux J, Haeberli P, Smithies O (1984) A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res 12:387–395
Dye DW, Bradbury JF, Goto M, Hayward AC, Lelliott RA, Schroth MN (1980) International standards for naming pathovars of phytopathogenic bacteria and a list of pathovar names and pathotype strains. Rev Plant Pathol 59:153–168
Goldberg I, Hochman A (1989) Purification and characterization of a novel type of catalase from the bacterium Klebsiella pneumoniae. Biochim Biophys Acta 991:330–336
Haas A, Brehm K, Kreft J, Goebel W (1991) Cloning, characterization, and expression in Escherichia coli of a gene encoding Listeria seeligeri catalase, a bacterial enzyme highly homologous to mammalian catalases. J Bacteriol 173:5159–5167
Hawley DK, McClure WR (1983) Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 11:2237–2255
Hedrick JL, Smith AJ (1968) Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys 128:155–164
Heym B, Zhang Y, Poulet S, Young D, Cole ST (1993) Characterization of the katG gene encoding a catalase-peroxidase required for the ioniazid susceptibility of Mycobacterium tuberculosis. J Bacteriol 175:4255–4259
Katsuwon J (1993) Characterization of catalases from a root-colonizing bacterium Pseudomonas putida. Ph D Thesis Utah State University, Utah, USA
Katsuwon J, Anderson AJ (1989) Responses of plant-colonizing pseudomonads to hydrogen peroxide. Appl Environ Microbiol 55:2985–2989
Katsuwon J, Anderson AJ (1992) Characterization of catalase activities in a root-colonizing isolate of Pseudomonas putida. Can J Microbiol 38:1026–1032
Keppler DL, Baker CJ, Atkinson MM (1989) Active oxygen production during bacteria-induced hypersensitive reaction in tobacco suspension cells. Phytopathology 79:974–978
Klotz MG (1993) The importance of bacterial growth phase for in planta virulence and pathogenicity testing: coordinated stress response regulation in fluorescent pseudomonads? Can J Microbiol 39:948–957
Klotz MG, Anderson AJ (1994) The role of catalase isozymes in the culturability of the root colonizer Pseudomonas putida after exposure to hydrogen peroxide and antibiotics. Can J Microbiol 40:382–387
Klotz MG, Hutcheson SW (1992) Multiple periplasmic catalases in phytopathogenic strains of Pseudomonas syringae. Appl Environ Microbiol 58:2468–2473
Knauf HJ, Vogel RF, Hammes WP (1992) Cloning, sequence, and phenotypic expression of katA, which encodes the catalase of Lactobacillus sake LTH677. Appl Environ Microbiol 58:832–839
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Loewen PC, Stauffer GV (1990) Nucleotide sequence of katG of Salmonella typhimurium LT2 and characterization of its product, hydroperoxidase I. Mol Gen Genet 224:147–151
Loprasert S, Negoro S, Okada H (1988) Thermostable peroxidase from Bacillus stearothermophilus. J Gen Microbiol 134:1971–1976
Margoliash E, Novogrodski A, Schejter A (1960) Irreversible reaction of 3-amino-1:2:4-triazole and related inhibitors with the protein of catalase. Biochem J 74:339–350
Morris SL, Nair J, Rouse DA (1992) The catalase-peroxidase of Mycobacterium intracellulare: nucleotide sequence analysis and expression in Escherichia coli. J Gen Microbiol 138:2363–2370
Murshudov GN, Melik-Adamyan WR, Grebenkow AI, Barynin VV, Vagin AA, Vainshtein BK, Dauter Z, Wilson KS (1992) Three-dimensional structure of catalase from Micrococcus lysodeikticus at 1.5 Å resolution. FEBS Lett 312:127–131
Nadler V, Goldberg I, Hochman A (1986) Comparative study of bacterial catalases. Biochim Biophys Acta 882:234–241
Platt T (1986) Transcription termination and the regulation of gene expression. Annu Rev Biochem 55:339–372
Pugsley AP (1993) The complete secretory pathway in gram-negative bacteria. Microbiol Rev 57:50–108
Ossowski I von, Mulvey MR, Leco PA, Borys A, Loewen PC (1991) Nucleotide sequence of Escherichia coli katE, which encodes catalase HPII. J Bacteriol 173:514–520
Ossowski I von, Hausner G, Loewen PC (1993) Molecular evolutionary analysis based on the amino acid sequence of catalase. J Mol Evol 37:71–76
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. A la laboratory manual, 2nd Cold String Harbor Press, Cold Spring Harbor, NY
Scandalios JG (1993) Oxygen stress and superoxide dismutases. Plant Physiol 101:7–12
Staskawicz BJ, Dahlbeck D, Keen NT, Napoli C (1987) Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J Bacteriol 169:5789–5794
Sutherland MW (1991) The generation of oxygen radicals during host plant responses to infection. Physiol Mol Plant Pathol 39:79–93
Triggs-Raine BL, Doble BW, Mulvey MR, Sorby PA, Loewen PC (1988) Nucleotide sequence of katG, encoding catalase HPI of Escherichia coli. J Bacteriol 170:4415–4419
Sha Z, Stabel TJ, Mayfield JE (1994) J Bacteriol 176:7375–7377
Author information
Authors and Affiliations
Additional information
Paper no. 4552 of the Utah Agricultural Experiment station
Rights and permissions
About this article
Cite this article
Klotz, M.G., Kim, Y.C., Katsuwon, J. et al. Cloning, characterization and phenotypic expression in Escherichia coli of catF, which encodes the catalytic subunit of catalase isozyme CatF of Pseudomonas syringae . Appl Microbiol Biotechnol 43, 656–666 (1995). https://doi.org/10.1007/BF00164770
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00164770