Abstract
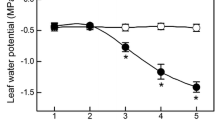
Gas exchange and abscisic acid content of Digitalis lanata EHRH. have been examined at different levels of plant water stress. Net photosynthesis, transpiration and conductance of attached leaves declined rapidly at first, then more slowly following the withholding of irrigation. The intercellular partial pressure of CO2 decreased slightly. The concentration of 2-cis(S)ABA increased about eight-fold in the leaves of non-irrigated plants as compared with well-watered controls. A close linear correlation was found between the ABA content of the leaves and their conductance on a leaf area basis. In contrast, the plot of net assimilation versus ABA concentration was curvilinear, leading to an increased efficiency of water use during stress. After rewatering, photosynthesis reached control values earlier than transpiration, leaf conductance and ABA content. From these data it is concluded that transpiration through the stomata is directly controlled by the ABA content, whereas net photosynthesis is influenced additionally by other factors.
Possible reasons for the responses of photosynthesis and water use efficiency to different stress and ABA levels are discussed.
Similar content being viewed by others

Abbreviations
- A:
-
net CO2 assimilation
- ABA:
-
abscisic acid
- Ci :
-
intercellular CO2 concentration
- g:
-
stomatal conductance
- T:
-
transpiration
- WUE:
-
water use efficiency
References
Bunce JA (1987) Species-specific responses to water stress of gas exchange parameters mimicked by applied abscisic acid. Can J Bot 65: 103–106
Cornic G & Miginiac E (1983) Nonstomatal inhibition of net CO2 uptake by abscisic acid in Pharbitis nil. Plant Physiol 73: 529–533
Cornish K & Zeevaart JAD (1984) Abscisic acid metabolism in relation to water stress and leaf age in Xanthium strumarium. Plant Physiol 76: 1029–1035
Cowan IR, Raven JA, Hartung W & Farquhar GD (1982) A possible role for abscisic acid in coupling stomatal conductance and photosynthetic carbon metabolism in leaves. Aust J Plant Physiol 9: 489–498
Dörffling K (ed) (1982) Das Hormonsystem der Pflanzen. Thieme, Stuttgart New York
Downton WJS, Loveys BR & Grant WJR (1988) Stomatal closure fully accounts for the inhibition of photosynthesis by abscisic acid. New Phytol 108: 263–266
Fischer E, Raschke K & Stitt M (1986) Effects of abscisic acid on photosynthesis in whole leaves: changes in CO2 assimilation, levels of carbon-reduction-cycle intermediates, and activity of ribulose-1,5-bisphosphate carboxylase. Planta 169: 536–545
Henson IE (1982) Abscisic acid and water relations of Rice (Oryza sativa L.): Sequential responses to water stress in the leaf. Ann Bot 50: 9–24
Johnson RC, Mornhinweg DW, Ferris DM & Heitholt JJ (1987) Leaf photosynthesis and conductance of selected Triticum species at different water potentials. Plant Physiol 83: 1014–1017
Kramer PJ (ed) (1983) Water relations of plants. Academic Press, New York
Krampitz MJ, Klug K & Fock HP (1984) Rates of photosynthetic CO2 uptake, photorespiratory CO2 evolution and dark respiration in water-stressed sunflower and bean leaves. Photosynthetica 18: 322–328
Mertens R, Deus-Neumann B & Weiler EW (1983) Monoclonal antibodies for the detection and quantitation of the endogenous plant growth regulator, abscisic acid. FEBS Letters 160: 269–272
Milborrow BV (1978) Abscisic acid. In: Phytohormones and related compounds — A comprehensive treatise, Vol. 1,. pp295–347, Letham, Goodwin, Higgins, (eds) Elsevier/North-Holland Biomedical Press, Amsterdam
Raschke K & Hedrich R (1985) Simultaneous and independent effects of abscisic acid on stomata and the photosynthesis apparatus in whole leaves. Planta 163: 105–118
Scholander PF, Hammel HT, Bradstreet ED & Hemmingsen EA (1965) Sap pressure in vascular plants. Science 148: 339–346
Stuhlfauth T, Klug K & Fock HP (1987) The production of secondary metabolites by Digitalis lanata during CO2 enrichment and water stress. Phytochemistry 26: 2753–2739
Stuhlfauth T, Sültemeyer DF, Weinz S & Fock HP (1988) Fluorescence quenching and gas exchange in a water stressed C3 plant, Digitalis lanata. Plant Physiol 86: 246–250
Tenhunen JD, Lange OL, Gebel J, Beyschlag W & Weber JA (1984) Changes in photosynthetic capacity, carboxylation efficiency, and CO2 compensation point associated with midday stomatal closure and midday depression of net CO2 exchange of leaves of Quercus suber. Planta 162: 193–203
Ward DA & Bunce JA (1987) Abscisic acid simultaneously decreases carboxylation efficiency and quantum yield in attached soybean leaves. J Exp Bot 38: 1182–1192
Ward DA & Drake BG (1988) Osmotic stress temporarily reverses the inhibitions of photosynthesis and stomatal conductance by abscisic acid-evidence that abscisic acid induces a localized closure of stomata in intact, detached leaves. J Exp Bot 39: 147–155
Weiler EW (1979) Radioimmunoassay for the determination of free and conjugated abscisic acid. Planta 144: 255–263
Weiler EW (1980) Radioimmunoassays for the differential and direct analysis of free and conjugated abscisic acid in plant extracts. Planta 148: 262–272
Weiler EW (1982) An enzyme-immunoassay for cis-(+)-abscisic acid. Physiol Plant 54: 510–514
Wong SC, Cowan IR & Farquhar GD (1978) Leaf conductance in relation to assimilation in Eucalyptus pauciflora Sieb. ex Spreng. Plant Physiol 62: 670–674
Wong SC (1980) Effects of elevated partial pressure of CO2 on rate of CO2 assimilation and water use efficiency in plants. In: Carbon dioxide and climate. Australian Research. Symposium Canberra 1980, Pearman GI (ed) pp 159–166
Zeevaart JAD & Boyer GL (1984) Accumulation and transport of abscisic acid and its metabolites in Ricinus and Xanthium. Plant Physiol 74: 934–939
Zhang J, Schurr U & Davies WJ (1987) Control of stomatal behaviour by abscisic acid which apparently originates in the roots J Exp Bot 38: 1174–1181
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Steuer, B., Stuhlfauth, T. & Fock, H.P. The efficiency of water use in water stressed plants is increased due to ABA induced stomatal closure. Photosynth Res 18, 327–336 (1988). https://doi.org/10.1007/BF00034837
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00034837