Abstract
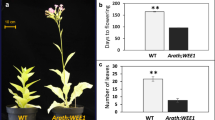
We investigated the effects of expressing a cDNA of cdc25, a mitotic inducer gene of Schizosaccharomyces pombe, on the development of transgenic tobacco plants (Nicotiana tabacum cv. Samsun). Nine independent primary transformants were regenerated containing the cdc25 sequence under the control of a cauliflower mosaic virus 35S gene promoter. Eight of the nine plants showed altered leaf morphology, the lamina being lengthened and twisted and the interveinal regions being pocketed. One of these was sacrificed for analysis of the root meristem, where the cells were found to be significantly smaller than in the wild type. The other seven were grown on and showed precocious flowering, flowers being produced earlier and in significantly greater numbers than in the wild type. They also developed abnormal flowers on short stalks developing in a position normally occupied by the most proximal axillary bud of otherwise normal flower pedicels. The presence or absence of these phenotypes in the primary transformants and in the T2 generation was associated with the presence or absence of detectable levels of cdc25 transcripts.
Similar content being viewed by others
References
Armstrong SW, Francis D: Differences in cell cycle duration of sister cells in secondary root meristems of Cocos nucifera L. Ann Bot 56: 803–813 (1985).
Avery GSJr: Structure and development of the tobacco leaf. Am J Bot 20: 565–592 (1933).
Bevan M: Binary Agrobacterium vectors for plant transformation. Nucl Acids Res 12: 8711–8721 (1984).
Conger AD, Fairchild LM: A quick-freeze method for making smear slides permanent. Stain Technol 28: 281–283 (1953).
Dunphy WG, Newport JW: Unravelling of mitotic control mechanisms. Cell 55: 925–928 (1988).
Edgar BA, O'Farrell PH: Genetic control of cell division patterns in the Drosophila embryo. Cell 57: 177–187 (1989).
Featherstone C, Russell P: Fission yeast p107weel mitotic inhibitor is a tyrosine/serine kinase. Nature 349: 808–811 (1991).
Feiler HS, Jacobs TW: Cell division in higher plants: a cdc gene, its 34 kDa product and histone H1 kinase activity in pea. Proc Natl Acad Sci USA 87: 5297–5401 (1990).
Ferreirra PCG, Hemerly AS, Villaroel R, Montagu MV, Inze D: The Arabidopsis functional homologue of the p34 cdc2 protein kinase. Plant Cell 3: 531–540 (1991).
Gould KL, Nurse P: Tyrosine phosphorylation of the fission yeast cdc2 + protein kinase regulates entry into mitosis. Nature 342: 39–45 (1989).
Gurr SJ, MacPherson MJ: PCR-directed cDNA libraries. In: MacPherson MJ, Quirke P, Taylor GR (eds) PCR: A Practical Approach, pp. 147–170. IRL Press, Oxford (1991).
Hata S, Kouchi H, Suzuka I, Ishii T: Isolation and characterization of cDNA clones for plant cyclins. EMBO J 10: 2681–2688 (1991).
Hemerle A, Bergounioux C, VanMontagu M, Inze D, Ferreira P: Genes regulating the plant cell cycle: Isolation of a mitotic-like cyclin from Arabidopsis thaliana. Proc Natl Acad Sci USA 89: 3295–3299 (1992).
Hirt H, Pay A, Gyorgyey J, Backo L, Nemeth K, Bogre L, Schweyen RJ, Heberle-Bors E, Dudits D: Complementation of a yeast cell cycle mutant by an alfalfa cDNA encoding a protein kinase homologous to p34cdc2. Proc Natl Acad Sci USA 88: 1636–1640 (1991).
Horsch RB, Fry JF, Hoffman NL, Eichholtz D, Rogers SG, Frayley RT: A simple and general method for transferring genes into plants. Science 227: 1229–1231 (1985).
Imajuku Y, Hirayama T, Endoh H, Oka A: Exon-intron organisation of the Arabidopsis thaliana protein kinase genes CDC2a and CDC2b. FEBS Lett 304: 73–77 (1992).
Kreis M, Shewry PR, Forde BG, Rahman S, Miflin BJ: Molecular analysis of a mutation conferring the high-lysine phenotype on the grain of barley (Hordeum vulgare). Cell 34: 161–167 (1983).
Kumagai A, Dunphy WG: The cdc25 protein controls tyrosine dephosphorylation of the cdc2 protein in a cell-free system. Cell 64: 903–914 (1991).
Lewin B: Driving the cell cycle: M phase kinase, its partners, and substrates. Cell 61: 743–752 (1990).
Lundgren K, Walworth N, Booher R, Dembski M, Kirschner M, Beach D: mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell 64: 1111–1122 (1991).
Lyndon RF, Cunningham ME: Control of shoot apical development via cell division. In: Trewavas AJ, Jennings B (eds) Plasticity in Plants: 40th Symposium of the Society for Experimental Biology, pp. 233–255. Company of Biologists, Cambridge (1986).
Lyndon RF, Francis D: The response of the shoot apex to light-generated signals from the leaves. In: Vince-Price D, Thomas B, Cockshull KE (eds) Light and the Flowering Process, pp. 77–85. Academic Press, London (1984).
Maksymowych R: Analysis of leaf development. Development and Cell Biology Series, Volume 1. Cambridge University Press, Cambridge (1973).
Marris C, Gallois P, Copley J, Kreis M: The 5′ flanking region of a barley B hordein gene controls tissue and developmental specific CAT expression in tobacco plants. Plant Mol Biol 10: 359–366 (1988).
McDaniel CN: Determination of growth pattern in axillary buds of Nicotiana tabacum. Devel Biol 66: 250–255 (1978).
Moreno S, Hayles J, Nurse P: Regulation of p34cdc2 protein kinase during mitosis. Cell 58: 361–372 (1989).
Moreno S, Nurse P, Russell P: Regulation of mitosis by cyclic accumulation of p80cdc25-mitotic inducer in fission yeast. Nature 34: 549–552 (1990).
Murashige T, Skoog F: A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 (1962).
Murray AW, Kirschner MW: Dominoes and clocks: The union of two views of the cell cycle. Science 246: 614–621 (1989).
Nurse P: Universal control mechanisms regulating onset of M-phase. Nature 344: 503–508 (1990).
Odell JT, Nagy F, Chua NH: Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. Nature 313: 810–812 (1985).
Russell P, Moreno S, Reed SI: Conservation of mitotic controls in fission and budding yeasts. Cell 57: 295–303 (1989).
Russell P, Nurse P: cdc25 functions as an inducer in the mitotic control of fission yeast. Cell 45: 145–153 (1986).
Sadhu K, Reed SI, Richardson H, Russell P: Human homologue of fission yeast cdc25 mitotic inducer is predominantly expressed in G2. Proc Natl Acad Sci USA 87: 5139–5143 (1990).
Shen W-J, Forde BG: Efficient transformation of Agrobacterium spp. by high voltage electroporation. Nucl Acids Res 17: 8385 (1989).
Silcock DJ, Francis D, Bryant JA, Hughes SG: Changes in nuclear DNA content, cell and nuclear size, and frequency of cell division in the cotyledons of Brassica napus L. during embryogenesis. J Exp Bot 41: 401–407 (1990).
Sokal RR, Rohlf FJ: Biometry, 2nd edition. WH Freeman & Co, San Francisco (1981).
Southern EM: Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98: 503–517 (1975).
Yanish-Perron C, Vieira J, Messing J: Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13 mp18 and pUC19 vectors. Gene 33: 103–119 (1985).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bell, M.H., Halford, N.G., Ormrod, J.C. et al. Tobacco plants transformed with cdc25, a mitotic inducer gene from fission yeast. Plant Mol Biol 23, 445–451 (1993). https://doi.org/10.1007/BF00019293
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00019293



