Abstract
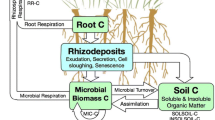
As global CO2 levels rise, can soils store more carbon and so buffer atmospheric CO2 levels? Answering this question requires a knowledge of the rates of C inputs to soil and of CO2 outputs via decomposition. Below-ground inputs from roots are a major component of the C flow into soils but are still poorly understood. In this article, new techniques for measuring rhizodeposition are reviewed and discussed and the need for cross-comparisons between methods is identified. One component of rhizodeposition, root exudation, is examined in more detail and evidence is presented which suggests that current estimates of exudate flow into soils are incorrect. A mechanistic mathematical model is used to explore how exudate flows might change under elevated CO2.
Similar content being viewed by others
References
Arnone, J A and Korner, C 1995 Soil and biomass carbon pools in model communities of tropical plants under elevated CO2. Oecologia 104, 61–71.
Azaizeh, H A, Marschner, H, Romheld, V and Wittenmayer, L 1995 Effects of a vesiculararbuscular mycorrhizal fungus and soil micro-organisms on growth, mineral nutrient acquisition and root exudation of soil-grown maize plants. Mycorrhiza 5, 321–327.
Barber, S A 1984 Soil Nutrient Bioavailability: a mechanistic Approach. John Wiley and Sons, New York, USA.
Barber, D A and Martin, J K 1976 The release of organic substances by cereal roots into soil. New Phytol. 76, 69–80.
Billes, G, Rouhier, H and Bottner, P 1993 Modifications of the carbon and nitrogen allocations in the plant (Triticum aestivum L.) soil system in response to increased atmospheric CO2 concentration. Plant and Soil 157, 215–225.
Boeuftremblay, V, Plantureux, S and Guckert, A 1995 Influence of mechanical impedance on root exudation of maize seedlings at 2 development stages. Plant and Soil 172, 279–287.
Bremer, E and Kuikman, P 1994 Microbial utilization of 14C[U]glucose in soil is affected by the amount and timing of glucose additions. Soil Biol. Biochem. 26, 511–517.
Cardon Z G 1996 Influence of rhizodeposition under elevated CO2 on plant nutrition and soil organic matter. Plant and Soil 187.
ChapinIII, F S, Moilanen, L and Kielland, K 1993 Preferential use of organic nitrogen for growth by a non-mycorrhizal arctic sedge. Nature 361, 151–153.
Cheng, W, Coleman, D C, Carroll, C R and Hoffman, C A 1993 In situ measurement of root respiration and soluble C concentration in the rhizosphere. Soil Biol. Biochem. 25, 1189–1196.
Chomba, B M, Guy, P D and Weger, H G 1993 Carbohydrate reserve accumulation and depletion in Engelmann spruce (Picea engelmannii parry) — effects of cold-storage and prestorage CO2, enrichment. Tree Physiol. 13, 351–364.
Christensen, H and Jakobsen, I 1993 Reduction of bacterial-growth by a vesicular-arbuscular mycorrhizal fungus in the rhizosphere of cucumber (Cucumis sativus L). Biol. Fert. Soils 15, 253–258.
Curl, E A and Truelove, B 1986 The Rhizosphere. Advanced Series in Agricultural Sciences 15. Springer-Verlag, New York, USA.
Darrah, P R 1991 Models of the rhizosphere. 1. Microbial-population dynamics around a root releasing soluble and insoluble carbon. Plant and Soil 133, 187–199
Fitter A H, Self G K, Wolfenden J, van Vuuren M M I, Brown T K, Williamson L, Graves J D and Robinson D 1996 Root production and mortality under elevated atmospheric CO2. Plant and Soil 187.
Fogel, R and unt, J 1983 Contribution of mycorrhizae and soil fungi to nutrient cycling in a Douglae-fir ecosystem. Can. J. For. Res. 13, 219–232.
Glandorf, D C M, Peters, L G L, van derSluis, I, Bakker, P A H M and Schippers, B 1993 Crop specificity of Rhizosphere pseudomonads and the involvement of root agglutinins. Soil Biol. Biochem. 25, 981–989.
Gifford, R M 1994 The global carbon-cycle — a viewpoint on the missing sink. Austr. J. Plant Physiol. 21, 1–15.
Harder, W and Dijkhuizen, L 1982 Strategies of mixed substrate utilization in microorganisms. Phil. Trans. R. Soc. Lond. B 297, 459–480.
Helal, H M and Sauerbeck, D R 1991 Short-term determination of the actual respiration rate of intact plant roots. In Plant roots and their environments. Eds. B LMcMichael and HPersson. pp 88–92. Elsevier, Amsterdam, the Netherlands.
Ineson P, Cotrufo M F, Bol R, Harkeness D D and Hartwig U 1996 Quantification of soil carbon inputs under elevated carbon dioxide: C3 plants in a C4 soil. Plant and Soil (In press).
Johansson, G 1992 Release of Organic-C from growing roots of meadow fescue (Festuca pratensis L.). Soil Biol. Biochem. 24, 427–433.
Johnen B G and Sauerbeck D R 1977 A tracer technique for measuring growth, mass and microbial breakdown of plant roots during vegetation. In Soil Organisms as Components of Ecosystems. Eds. V Lohm and T Persson. pp 366–373. Proc VI Int. Soil Zoo. Coll. Ecol. Bull. Stockholm, 25.
Jones, D L and Darrah, P R 1992 Resorption of organic-components by roots of Zea mays L. and its consequences in the rhizosphere. 1. Resorption of 14C-labeled g'ucose, mannose and citric-acid. Plant and Soil 143, 259–266.
Jones, D L and Darrah, P R 1993a Re-sorption of organic-compounds by roots of Zea mays L. and its consequences in the rhizosphere. 2. Experimental and model evidence for simultaneous exudation and re-sorption of soluble C compounds. Plant and Soil 153, 47–59.
Jones, D L and Darrah, P R 1993b Influx and efflux of amino-acids from Zea mays L. roots and their implications for N-nutrition and the rhizosphere. Plant and Soil 156, 87–90.
Jones, D L and Darrah, P R 1994 Amino-acid influx at the soil-root interface of Zea mays L. and its implications in the rhizosphere. Plant and Soil 163, 1–12.
Jones, D L and Darrah, P R 1995 Influx and efflux of organic-acids across the soil-root interface of Zea mays L. and its implications in rhizosphere C flow. Plant and Soil 173, 103–109.
Kraffczyk, I, Trolldeniner, G and beringer, H 1984 Soluble root exudates of maize: influence of potassium supply and rhizosphere micro-organisms. Soil Biol. Biochem. 16, 315–322.
Lamari, L and Sabaou, N 1993 Comparative-analysis of rhizosphere bacterial-flora of date palm cultivars resistant and susceptible to Fusariosis. Can J. Microbiol. 39, 874–881.
Lambers, H 1987 Growth, respiration, exudation and symbiotic associations: the fate of carbon translocated to the roots. In Root development and function. Eds. P JGregory, J VLake and D ARose. pp 125–146. Soc. Exp. Biol. Seminar Ser. 30. Cambridge University Press Cambridge, UK.
Lerouge, P 1994 Symbiotic host-specificity between leguminous plants and rhizobia is determined by substituted and acylated glucosamine oligosaccharide signals. Glycobiology 4, 127–134.
Lewis, J D, Thomas, R B and Strain, B R 1994 Effect of elevated CO2 on mycorrhizal colonization of lobelly pine (Pinus taeda L.) seedlings. Plant and Soil 165, 81–88.
Liljeroth, E 1995 Comparisons of early root cortical senescence between barley cultivars, Triticum species and other cereals. New Phystol. 130, 495–501.
Mahall, B E and Callaway, R M 1992 Root communication mechanisms and intracommunity distributions of 2 Mojave desert shrubs. Ecology 73, 2145–2151.
Martin, J K and Merckx, R 1992 The partitioning of photosynthetically fixed carbon within the rhizosphere of mature wheat. Soil Biol. Biochem. 24, 1147–1156.
Marschner, H 1995 Mineral Nutrition of higher Plants. 2nd Ed. Academic Press, London, UK.
Mavingui, P, Laguerre, G, Berge, O and Eleulin, T 1992 Genetic and phenotypic diversity of Bacillus polymyxa, in soil and in the wheat rhizosphere. Appl. Environ. Microbiol. 58, 1894–1903.
McCully, M E 1987 Selected aspects of the structure and development of field grown roots with special reference to maize. In Root Development and Function. Eds. P JGregory, J VLake and D ARose. pp 53–70 Soc. Exp. Biol. Seminar Ser. 30. Cambridge University Press Cambridge, UK.
McDougall, B M and Rovira, A D 1970 Sites of exudation of 14C-labelled compounds from wheat roots. New Phytol. 69, 999–1003.
Meharg, A A 1994 A critical review of labelling techniques used to quantify rhizosphere carbonflow. Plant and Soil 166, 55–62.
Meharg, A A and Killham, K 1995 Loss of exudates from the roots of perennial ryegrass inoculated with a range of microorganisms. Plant and Soil 170, 345–349.
Mozafar, A 1991 Contact with ballotini (glass spheres) stimulates exudation of iron-reducing an iron-chelating substances from barley roots. Plant and Soil 130, 105–108.
Mozafar, A, Duss, F and Oertli, J J 1992 Effect of Pseudomonas fluorescens on the root exudates of 2 tomato mutants differently sensitive to Fe chlorosis. Plant and Soil 144, 167–176.
Muhling, K H, Schubert, S and Mengel, K 1993 Mechanism of sugar retention by roots of intact maize and bean-plants. Plant and Soil 156, 99–102.
Muhling, K H, Schubert, S and Mengel, K 1993 Role of plasmalemma H+-ATPase in sugar retention by roots of intact maize and field bean-plants. Z. Pflanzenernahr. Bodenkd. 15i5, 155–161.
Muller, S, Vandermerwe, A, Schildknecht, H and Visser, J H 1993 An automated-system for large-scale recovery of germination stimulants and other root exudates. Weed Sci. 41, 138–143.
Nielsen, K L, Lynch, J P, Jablokow, A G and Curtis, P S 1994 Carbon cost of root systems: an architectural approach. Plant and Soil 165, 161–169.
Norb, R J, ONeill, Hood, W G and Luxmore, R J 1987 Carbon allocation, root exudation and mycorrhizal colonization of Pinus echinata seedlings grown under CO2 enrichment. Tree Physiol. 3, 203–210.
Norby, R J 1994 Issues and perspectives for investigating root responses to elevated atmospheric carbon dioxide. Plant and Soil 165, 9–20.
O'Keefe, D M and Sylvia, D M 1992 Chronology and mechanisms of P uptake by mycorrhizal sweet potato plants. New Phytol. 122, 651–659.
Pellet D M, Grunes D L and Kochian L V Organic acid exudation as an aluminium tolerance mechanism in maize (Zea mays L.). Planta 196, 788–795.
Posta, K, Marschner, H and Romheld, V 1994 Manganese reduction in the rhizosphere of mycorrhizal and non-mycorrhizal maize. Mycorrhiza 5, 119–124.
Powlson, D S 1994 The soil microbial biomass: before, beyond and back. In Beyond the Biomass: Compositional and Functional Analysis of Soil Microbial Commumities. Eds. KRitz, JDightom and K EGiller. John Wiley and Sons, Chichester, UK.
Rogers, H H, Runion, G B and Krupa, S V 1994 Plant responses to atmospheric CO2 enrichment with emphasis on roots and the rhizosphere. Environ Pollut. 83, 155–189.
Shepherd, T and Davies, H V 1994a Patterns of short-term amino-acid accumulation and loss in the root-zone of liquid-cultured forage rape (Brassica napus L.). Plant and Soil 158, 99–109.
Shepherd, T and Davies, H V 1994b Effect of exogenous amino-acids, glucose arid citric-acid on patterns of short-terms accumulation and loss of amino-acid from the root-zone of sand-cultured forage rape (Brassica napus L.). Plant and Soil 158, 111–118.
Stacey, G, Sanjuan, J, Luka, S, Dockendorff, T and Carlson, R W 1995 Signal exchange in the Bradyrhizobium-soybean symbiosis. Soil Biol. Biochem. 27, 473–483.
Swinnen, J 1994 Evaluation of the use of a model rhizodeposition technique to separate root and microbial respiration in soil. Plant and Soil 165, 89–104.
Swinnen, J, vanVeen, J A and Merckx, R 1994 Rhizosphere carbon fluxes in field-grown spring wheat: model calculations based on 14C partitioning after pulse-labelling. Soil Biol. Biochem. 26, 171–182.
Swinnen, J, vanVeen, J A and Merckx, R 1995 Carbon fluxes in the rhizosphere of winter-wheat and spring barley with conventional vs. integrated farming. Soil Biol. Biochem. 27, 811–820.
Trofymow, J A, Coleman, D C and Cambardella, C 1987 Rates of rhizodeposition and ammonium depletion in the rhizosphere of axenic oat roots. Plant and Soil 97, 333–344.
Vanoverbeek, L S and Vanelsas, J D 1995 Root exudate-induced promoter activity in Pseudomonas fluorescens mutants in the wheat rhizosphere. Appl. Environ. Microbiol. 61, 890–898.
Waschkie, C, Schropp, A and Marschner, H 1994 Colonization of grapevine (Vitis sp) by fluorescent pseudomonads and endomycorrhizal fungi. Plant and Soil 162, 219–227.
Waschutza, S. Hofmann, N, Niemann, E G and Fendrik, I 1992 Investigations on root exudates of Korean rice. Symbiosis 13, 181–189.
Whipps, J M 1985 Effect of CO2 concentration on growth carbon distribution and loss of carbon from the roots of maize. J. Exp. Bot. 36, 644–651.
Whipps, J M 1990 Carbon economy. In The Rhizosphere. Ed. J M Lynch. pp 59–98. Wiley Interscience/John Wiley and Sons, Chichester, UK.
Wong, S C 1990 Elevated atmospheric partial-pressure of CO2 and plant-growth. 2. Nonstructural carbohydrate content in cotton plants and its effect on growth-parameters. Photosynthes Res. 23, 171–180.
Yu, J Q and Matsui, Y 1994 Phytotoxic substances in root exiidates of cucumber (Cucumis sativus L.). J. Chem. Ecol. 20, 21–31.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Darrah, P.R. Rhizodeposition under ambient and elevated CO2 levels. Plant Soil 187, 265–275 (1995). https://doi.org/10.1007/BF00017092
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00017092