Abstract
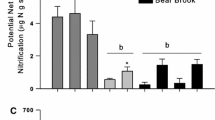
Seasonal patterns and annual rates of N inputs, outputs, and internal cycling were determined for an old-growth mixed-conifer forest floor in the Sierra Nevada Mountains of California. Rates of net N mineralization within the forest floor, and plant N-uptake and leaching of inorganic N from the forest floor were 13, 10, and 9 kg-N ha-1 yr-1, respectively. The Mediterranean-type climate appeared to have a significant effect on N cycling within this forest, such that all N-process and flow rates showed distrinct seasonal patterns. We estimated the forest floor supplies less than one-third of the total aboveground plant N-uptake in this forest. The rate of net nitrification within the forest floor was always low (1 kg-NO3 --N ha-1 30d-1). Mean residence times for organic matter and N in the forest floor were 13 and 34 years, respectively, suggesting that this forest floor layer is a site of net N immobilization within this ecosystem. We examined the influence of the forest floor on mineral soil N dynamics by injecting small amounts of15N-enriched (NH4)2SO4 solutions into the surface mineral soil with the forest floor present (+FF) or removed (-FF). K2SO4-extractable NO3 --N, total inorganic-N, and total-N pool sizes in the mineral soil were initially increased after forest floor removal (after 4 months), but NO3 --N and total inorganic-N were not significantly different thereafter. Microbial biomass-N and K2SO4-extractable total-N pool sizes were also found to be larger in mineral soils without a forest floor after 1 and 1.3 years, respectively. Total15N-recovery was greater in the +FF treatment compared to the -FF treatment after 1-year (about 50% and 35%, respectively) but did not differ after 1.3 years (both about 35%), suggesting that the forest floor delays but does not prevent the N-loss from the surface mineral soil of this forest. We estimated using our15N data that fungal translocation from the mineral soil to the forest floor may be as large as 9 kg-N ha-1 yr-1 (similar in magnitude to other N flows in this forest), and may account for all of the observed absolute increase of N in litter during the early stages of decomposition at this site. Our results suggest that the forest floor acts both as a source and sink for N in the mineral soil.
Similar content being viewed by others
References
Adams MA, Polglase PJ, Attiwill PM & Weston CJ (1989) In situ studies of nitrogen mineralizaiton and uptake in forest soils: some comments on methodology. Soil Biology and Biochemistry 21: 423–429
Berg B & Söderström B (1979) Fungal biomass and nitrogen in decomposing Scots pine needle litter. Biology and Biochemistry 11: 339–341
Binkley D & Hart SC (1989) The components of nitrogen availability assessments in forest soils. Advances in Soil Science 10: 57–112
Bocock KL (1963) Changes in the amount of nitrogen in decomposing leaf letter in sessile oak (Quercus petraea). Journal of Ecology 51: 555–556
Bremner JM & Mulvaney CS (1982) Nitrogen — Total In: Page AL, Miller RH & Keeney DR (Eds) Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties (pp 595–624). Second Edn. American Society of Agronomy, Madison, Wisconsin
Bringmark L (1980) Ion leaching through a podsol in a Scots pine stand. In: Persson T (Ed) Structure and Function of Northern Coniferous Forests — An Ecosystem Study. Ecological Bulletins (Stockholm) 32: 341–361
Brookes PC, Landman A, Pruden G & Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biology and Biochemistry 17: 837–842
Brooks PD, Stark JM, McInteer BB & Preston T (1989) A diffusion method to prepare soil extracts for automated Nitrogen-15 analysis. Soil Science Society of American Journal 53: 1707–1711
Cole DW (1981) Nitrogen uptake and translocation by forest ecosystems. Ecological Bulletins (Stockholm) 33: 219–232
Cole DW & Rapp M (1981) Elemental cycling in forest ecosystems. In: Reiche DE (Ed) Dynamic Properties of Forest Ecosystems (pp 341–409). International Biological Program, 23
Davidson EA, Eckert RW, Hart SC & Firestone MK (1989) Direct extraction of microbial biomass nitrogen from forest and grassland soils of California. Soil Biology and Biochemistry 21: 773–778
Davidson EA, Hart SC & Firestone MK (submitted) Nitrate is important in the internal nitrogen cycle of a mature coniferous forest. Ecology
DiStefano Jf & Gholz HL (1986) A proposed use of ion exchange resins to measure nitrogen mineralization and nitrification in intact soil cores. Communications in Soil Science and Plant Analysis 17: 989–998
Dyck WJ, Mees CA & Hodgkiss PD (1987) Nitrogen availability and comparison of uptake in two New Zealand Pinus radiata forests. New Zealand Journal of Forestry Science 17: 338–352
Ellenberg VH (1977) Stickstoff als Standortsfaktor, insbesondere fr Mitteleuropeische Pflanzengesellschaften. Oecologia Plantarum 12: 1–22
Eno CH (1960) Nitrate production in the field by incubating the soil in polyethylene bags. Soil Science Society of American Proceeding 24: 277–279
Fahey TL, Yavitt JB, Peasson JA & Knight DH (1985) The nitrogen cycle in lodgepole pine forests, southeastern Wyoming. Biogeochemistry 1: 257–275
Gessel SP, Cole DW & Steinbrenner EC (1973) Nitrogen balances in forest ecosystems of the pacific northwest. Soil Biology and Biochemistry 5: 19–34
Gosz JR, Likens GE & Bormann FH (1973) Nutrient releases from decomposing leaf and branch litter in the Bubbard Brook Forest, New Hampshire, Ecological Monographs 43: 173–191
Granhall U & Lindberg T (1977) Nitrogen fixation at coniferous forest sites within the Swecon project. Swedish Coniferous Forest Project Technical report 11: 1–39
Grier CC, Vogt KA, Keyes MR & Edmonds RL (1981) Biomass distribution and aboveand belowground production in young and mature Abies amabilis zone ecosystems of the Washington Cascades. Canadian Journal of Forest Research 11: 155–167
Hart SC (1990) Control of decomposition processes and nutrient flow in a California forest and grassland. Unpublished Ph.D. Dissertation, University of California, Berkeley
Hart SC & Binkley D (1984) Colorimetric interference and recovery of adsorbed ions from ion exchange resins. Communications in Soil Science and Plant Analysis 15: 893–902
Hart SC & Firestone MK (1989) Evaluation of three in situ soil nitrogen availability assays. Canadian Journal of Forest Research 19: 185–191
Hart SC & Gunther AJ (1989) In situ estimates of annual net nitrogen mineralization and nitrification in a subarctic watershed. Oecologia 80: 284–288
Jackson LE, Schimel JP & Fireston MK (1989) Short-term partitioning of ammonium and nitrate between plants and microbes in an annual grassland. Soil Biology and Biochemistry 21: 409–415
Jackson LE, Strauss RB, Fireston MK & Bartolome JW (1988) Plant and soil nitrogen dynamics in California annual grassland. Plant and Soil 110: 9–17
Jorgensen JR, Wells CG & Metz LZ (1980) Nutrient changes in decomposing loblolly pine forest floors. Soil Science Society of America Journal 44: 1307–1314
Keeney DR and Nelson DW (1982) Nitrogen — inorganic forms. In: Page AL, Miller RH & Keeney DR (Eds) Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties (pp 643–698). Second Edn. American Society of Agronomy, Madison, Wisconsin
Lamont BB (1983) Strategies for maximizing nutrient uptake in two Mediterranean ecosystems of low nutrient status. In: Kruger FJ, Mitchell DT & Jarvis JUM (Eds) Mediterranean-Type Ecosystems — The Role of Nutrients (pp 246–273). Ecological Studies 43, Springer-Verlag, Berlin
Nadelhoffer KJ, Aber JD & Melillo JM (1983) Leaf litter production and soil organic matter dynamics along a nitrogen-availability gradient in Southern Wisconsin. Canadian Journal of Forest Research 13: 12–21
Nadelhoffer KJ, Aber JD & Melillo JM (1984) Seasonal patterns of ammonium and nitrate uptake in nine temperate forest ecosystems. Plant and Soil 80: 321–335
Nadelhoffer KJ, Aber JD & Melillo JM (1985) Fine roots, net primary production, and soil nitrogen availability: a new hypothesis. Ecology 66: 1377–1390
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44: 322–331
Pastor J, Aber JD, McClaugherty CA & Melillo JM (1984) Aboveground production and N and P cycling along a nitrogen mineralization gradient on Blackhawk Island, Wisconsin. Ecology 65: 256–268
Powers RF (1990) Soil nitrogen mineralization along an altitudinal gradient: interactions of soil temperature, moisture, and substrate quality. Forest Ecology and Management 30: 19–29
QuikChem Systems (1986) QuikChem method no. 12-107-06-1-A. Quikchem Systems, division of Lachat Chemicals, Inc., Mequon, Wisconsin
QuikChem Systems (1987) QuikChem method no. 12-107-04-1-A. Quikchem Systems, division of Lachat Chemicals, Inc., Mequon, Wisconsin
Schimel JP & Firestone MK (1989) Nitrogen incorporation and flow through a coniferous forest soil profile. Soil Science Society of America Journal 53: 779–784
Schimel JP, Jackson LE & Firestone MK (1989) Spatial and temporal effects on plantmicrobial competition for inorganic nitrogen in a California annual grassland. Soil Biology and Biochemistry 21: 1059–1066
Smethurst PJ & Nambiar EKS (1989) An appraisal of the in situ soil-core technique for measuring nitrogen uptake by a young Pinus radiata plantation. Soil Biology and Biochemistry 21: 939–942
Sollins P & McCorison FM (1981) Nitrogen and carbon solution chemistry of an old growth coniferous forest watershed before and after cutting. Water Resources Research 17: 1409–1418
Staaf H & Berg B (1977) Mobilization of plant nutrients in a Scots pine forest mor in Central Sweden. Silva Fennica 11: 210–217
Steel RGD & Torrie JH (1980) Principles and Procedures of Statistics, 2nd edition. McGraw-Hill, New York, 633 pp
STSC, Inc. (1986) Statgraphics user's guide. STSC Inc., Rockville, MD
Vogt, KA, Grier CC, Meier CE & Keyes MR (1983) Organic matter production and nutrient dynamics in forest floors of young and mature Abies amabilis stands in western Washington, as affected by fine-root input. Ecological Monographs 53: 139–157
Vogt KA, Grier CC & Vogt DJ (1986) Production, turnover, and nutrient dynamics of above- and belowground detritus of world forests. Advances in Ecological Research 15: 303–377
Waring RH & Schlesinger WH (1985) Decomposition and forest soil development. In: Forest Ecosystems: Concepts and Management (pp 181–210). Academic Press, Inc., NY
Weber MG & Van Cleve K (1981) Nitrogen dynamics in the forest floor of interior Alaska black spruce ecosystems. Canadian Journal of Forest Research 11: 743–751
Weber MG & Van Cleve K (1984) Nitrogen transformations in feather moss and forest floor layers of interior Alaska black spruce ecosystems. Canadian Journal of Forest Research 14: 278–290
Wells C & Jorgensen JR (1975) Nutrient cycling in loblolly pine plantations. In: Bernier B & Winget CH (Eds) Forest Soils and Forest Land Management (pp 137–158). Les Presses de L'Universite Laval, Quebec
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hart, S.C., Firestone, M.K. Forest floor-mineral soil interactions in the internal nitrogen cycle of an old-growth forest. Biogeochemistry 12, 103–127 (1991). https://doi.org/10.1007/BF00001809
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00001809