Abstract
We report a case of a 25 year-old male with a functionally univentricular heart. He underwent consult for clearance prior to employment and has been asymptomatic since childhood with good functional capacity, his 6 minute walk test was normal at 300 meters. Pertinent physical findings were a right ventricular heave, a grade 3/6 systolic murmur over the 2nd-3rd intercostal spaces left parasternal border, a continuous murmur over the interscapular area and cyanosis of the nailbeds. Laboratory exams showed mild erythrocytosis and hypoxemia. Transthoracic and transesophageal echocardiographic findings showed a large ventricular septal defect (apical intraventricular septum remnant), atrioventricular discordance, malposition of the great arteries and severe pulmonary stenosis. Major aorto-pulmonary collaterals were demonstrated on hemodynamics study. Patient is stable and living a normal life but advised to avoid moderate to heavy strenuous activities. He is on a regular follow – up at our out patient clinic and there are no planned immediate surgical interventions at present.
Similar content being viewed by others
Introduction
Univentricular hearts (UVH) are a rare congenital occurrence, a New England Registry reported 54 cases per million livebirths.1 The survival of these patients depends on a balanced and controlled systemic and pulmonary circulation. Most of these patients are advised to undergo the staged Fontan procedure which has increased survival as pooled from case reports in the literature. Our patient belongs to the minority of these cases who did not undergo any surgical interventions and is living a normal life inspite of his conundrum of congenital heart anomalies.
Case description
A 25-year-old male consulted our outpatient clinic for pre-employment evaluation. He had a history of an unconfirmed congenital heart defect since birth with no reported symptoms of easy fatigability, shortness of breath, chest pain, syncope or cyanosis. He had no other co-morbid conditions, no family history of congenital diseases, cardiomyopathy, or sudden cardiac death. He is a non-smoker and non-alcoholic beverage drinker. Birth and maternal history were unremarkable, being born full term via vaginal delivery by a midwife in a hospital.
Physical examination revealed stable vital signs with a blood pressure of 90/60, and a heart rate of 72 beats per minute. Pertinent findings were the noted right ventricular heave, point of maximal impulse undisplaced and a harsh 3/6 systolic murmur over the 2nd–3rd intercostal space (ICS) left parasternal border. There were no signs congestion present but there was an evident 2/6 continuous murmur over the interscapular area. Pulses were full and equal and nailbeds of his fingers and his toes were cyanotic but there was no clubbing observed (Figure 1A and 1B). His 6-minute walk test was normal at 300 meters.
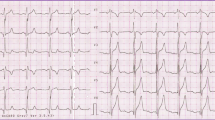
The ECG is Regular sinus rhythm, right axis deviation with biatrial and biventricular enlargement (Figure 1), while his chest x-ray showed levocardia and levoposition; cardiomegaly of LV form with a CT ratio of 0.54. Other laboratory exam results were of erythrocytosis with hemoglobin of 183 g/L, hematocrit of 0.542, and hypoxemia with a pO2 at 58 and oxygen saturations at 90% at room air. Creatinine and electrolyte values were normal.

Figure 3A

Figure 3B
The transthoracic (TTE) and transesophageal (TEE) 2D echocardiograms showed the subsequent findings:
-
Viscero-atrial situs solitus
-
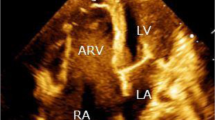
The morphologic left atrium drained all four pulmonary veins and was connected via the left sided tricuspid valve to the morphologic right ventricle (RV) (which was on the left). (Figure 4 )
-
The morphologic right atrium drained the superior and inferior vena cave and was connected via the right-sided mitral valve to the morphologic left ventricle (LV) (which was on the right). (Figure 4 )
-
There was a large ventricular septal defect with a remnant of the interventricular septum found at the apex. (Figure 4 )
-
Ventricles showed good contractility.
-
There was malposition of the great arteries with the aorta lying anterior to the pulmonary artery (PA). (Figure 5 )
-
There was severe supravalvar stenosis on doppler interrogation of the pulmonary artery. (Figure 6 )
-
The left sided tricuspid and right-sided mitral valve showed trivial regurgitation.
-
No major aortopulmonary collaterals (MAPCAs) conclusively demonstrated.
4-chamber view of the TTE depicting the large VSD, the left atrium connected to the inferiorly located tricuspid valve (note its septal attachment) and the right atrium connected to the mitral valve. (LV left ventricle, RV right ventricle, LA left atrium, RA right atrium, MV mitral valve, TV tricuspid valve)
We proceeded with cardiac catheterization for hemodynamic studies and the results were as follow:
-
1.
Oximetry findings: (Large Ventricular Septal Defect)
-
18% O2 step-up from SVC to PA
-
10% O2 step-up from RA to RV
-
5% O2 step- up from RV to PA
-
-
2.
Pressures: (Functional Univentricular Heart)
-
Equalization of RV and LV Pressure
-
Valvar/ Supravalvar Pulmonic Stenosis (Figure 7)
-
-
3.
Angiography:
Discussion
Univentricular hearts (UVH) are a rare congenital occurrence. Classification and nomenclature of UVH remains an unresolved issue, as some define it as one ventricular chamber that receives both AV valves or a common atrioventricular (AV) valve,1 while others simply state that it is when the entire AV junction is connected to a single ventricular chamber (thus including atretic AV valves).2
The case presented wherein there is a large ventricular septal defect present, although not strictly categorized as a single ventricle, can functionally be considered as one because of the large communication between ventricles, thus allowing mixing of ventricular inflow and making the two chambers act as a single ventricle. It is recognized in literature that huge ventricular septal defects in which most of the ventricular septum is absent, is considered one of the forms of a functionally univentricular heart but because of its septal apical remnant, the ventricular inlets are committed to recognizably separate apical ventricular components.3
For patients with a univentricular heart, prognosis is poor with only 30% expected to survive to the first year of life.4 Whether this holds true for those with functional UVH is not supported in literature but we can assume that the characteristics that affect its prognosis may be similar due to its similar hemodynamic profile. The patient despite his having a complex congenital heart disease remains asymptomatic for the past 25 years, and the explanation for this lies in the associated cardiac malformations he possesses. In a study by Hager et al…, 5 it described the most important characteristics of patients with UVH who may survive into late adulthood. This included transposition of the great arteries, absence of systemic outflow obstruction, adequately functioning AV valves and moderate pulmonary outflow obstruction. Of these features what seems to be critical in preventing a symptomatic state is the moderate pulmonary outflow obstruction as it will prevent early pulmonary congestion but at the expense of a certain degree of hypoxemia.1 All these characteristics were present in our patient to a certain extent, which may explain his relatively asymptomatic state. The mild cyanosis present only on the nailbeds on physical exam was not consistent with the finding of severe pulmonic stenosis on echo but adding the presence of a continuous murmur over the interscapular area led to the consideration of MAPCAs which was confirmed thru the hemodynamics study. These represent fetal primitive intersegmental arteries that originate from the descending aorta and have not involuted. They gain access to the lung through the hilum and connect with the native pulmonary arteries in the mediastinum or at the lobar or subsegmental level.3 These are presumed to supply the native pulmonary circulation.
Another unique finding in this case was the atrioventricular discordance, in which the morphologic RV was on the left while the morphologic LV was on the right. This could be also a case of congenitally corrected transposition of the great arteries (cc-TGA) that is difficult to recognize due to the presence of the large VSD. But this was answered by the results of the cardiac catheterization which confirmed that it was a functional univentricle.
This patient was not advised to undergo operation since he is asymptomatic with a balanced systemic and pulmonary circulation at present time, the severe pulmonic stenosis has protected his pulmonary circulation from volume overload and manipulating this might create decompensation of the patient. This case can be compared to the oldest reported case of univentricular heart in a 73 years old woman who had 3 children and lives a normal life in the United States as reported by Chen et al.8 She did not undergo any surgical intervention and her longevity can be explained by the presence of the severe subvalvular stenosis.
Conclusion
Univentricular heart is a conglomeration of rare and complex congenital heart diseases. Although most patients are advised staged surgical procedure, a minority of these patients who have a balance systemic and pulmonary circulation can be managed medically and be on close follow-up for any development of heart failure symptoms and arrhythmias.
Bibliography
Khairy P, Poirier N, Mercier LA. Univentricular Heart. Circulation 2007; 115:800– 812.
Anderson RH, Becker AE, Wilkinson JL. Proceedings: morphogenesis and nomenclature of univentricular hearts. Br Heart J. 1975; 37:781– 782.
Anderson HA, Baker EJ, Penny D, et al… Pedriatric Cardiology. 3rd edition; Elsevier; 2010.
Samanek M. Children with congenital heart disease: probability of natural survival. Pediatr Cardiol. 1992; 13:152– 8.
Hager A, Laemmerer H, Eicken A, Fratz S, Hess J et al… Long-term survival of patients with Univentricular heart not treated surgically. The Journal of Thoracic and Cardiovascular Surgery 2002; 123:1214– 1217.
Van Praagh R, Papagiannis, Grunenfelder J, et al… Pathologic anatomy of corrected transposition of the great arteries: Medical and surgical implications. Am Heart J 1998; 135: 772– 785.
Allwork SP, Bentall HH, Becker AE, et al… Congenitally corrected transposition of the great arteries: morphologic study of 32 cases. Am J Cardiol 1976; 38: 910– 923.
Michael, S. Chen; Adel, Younoszai; Hitinder, S. Gurm; Craig, R. Asher: Images in Cardiovascular Medicine: Univentricular Heart. Circulation. 2004; 109:2030.
Author information
Authors and Affiliations
Additional information

Cristine Catindig-Santelices, MD, FPCP The primary author is currently the Chief Fellow of the Section of Cardiology, University of the Philippines Philippine General Hospital (UP PGH). She finished her Doctor of Medicine at University of Santo Tomas (UST) Manila Philippines in 2005 and took her Internal Medicine residency at Manila Doctors Hospital where she served as Chief Resident in 2010. Dr. Santelices is a Fellow of Philippine College of Physicians and an Associate Fellow of Philippine Heart Association.
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Catindig-Santelices, C., Reyes, M., Matulac, M. et al. A Functional Univentricle: Large Ventricular Septal Defect, Atrioventricular Discordance, Malposition of the Great Arteries, Pulmonary Stenosis, and Major Aorto Pulmonary Collaterals. GSTF J Adv Med Res 1, 4 (2014). https://doi.org/10.7603/s40782-014-0004-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.7603/s40782-014-0004-y