Abstract
For more than 15 years, motor interference paradigms have been used to investigate the influence of action observation on action execution. Most research on so-called automatic imitation has focused on variables that play a modulating role or investigated potential confounding factors. Interestingly, furthermore, a number of functional magnetic resonance imaging (fMRI) studies have tried to shed light on the functional mechanisms and neural correlates involved in imitation inhibition. However, these fMRI studies, presumably due to poor temporal resolution, have primarily focused on high-level processes and have neglected the potential role of low-level motor and perceptual processes. In the current EEG study, we therefore aimed to disentangle the influence of low-level perceptual and motoric mechanisms from high-level cognitive mechanisms. We focused on potential congruency differences in the visual N190 − a component related to the processing of biological motion, the Readiness Potential − a component related to motor preparation, and the high-level P3 component. Interestingly, we detected congruency effects in each of these components, suggesting that the interference effect in an automatic imitation paradigm is not only related to high-level processes such as self-other distinction but also to more low-level influences of perception on action and action on perception. Moreover, we documented relationships of the neural effects with (autistic) behavior.
Similar content being viewed by others
Introduction
A plethora of studies have used the imitation inhibition paradigm (Brass, Bekkering, & Prinz, 2001; Brass et al., 2000; Brass, Derrfuss, Matthes-von Cramon, & von Cramon, 2003; Brass, Zysset, et al., 2001; Stürmer, Aschersleben, & Prinz, 2000) to investigate the automatic influence of observed behavior on own actions (for an extensive review, see Heyes, 2011). That is, as compared to a baseline trial, individuals react slower and make more errors when observing a movement that is incompatible with an own intended movement (incongruent trial), while they are faster when the observed movement is compatible with the intended movement (congruent trial). This behavioral congruency effect is what is referred to as the “motor interference effect.” Follow-up studies showed that the motor interference effect is largely distinct from spatial compatibility effects (Bertenthal, Longo, & Kosobud, 2006; Brass et al., 2000; Heyes, Bird, Johnson, & Haggard, 2005). In other words, the spatial correspondence between the own and the observed movement cannot (entirely) explain the motor interference effect. This led to the assumption that the observation of another’s action triggers a corresponding motor representation in the observer, which then interferes with one’s own action representation (Brass et al., 2003, 2005; Brass, Zysset, et al., 2001; Stürmer et al., 2000).
However, in principle, the interference effect in the imitation inhibition paradigm can be explained by at least three different processes, which are not mutually exclusive.
First, the participant’s motor preparation of the intended action could impact visual perception, as suggested by numerous theoretical accounts and studies (Brass & Heyes, 2005; Greenwald, 1970; Hommel, Müsseler, Aschersleben, & Prinz, 2001; Kühn, Keizer, Rombouts, & Hommel, 2011b; for a review, see Shin, Proctor, & Capaldi, 2010). Here the basic idea is that motor preparation involves an anticipation of the action effect, which could facilitate the visual processing of a compatible observed hand movement (with respect to a baseline and incongruent trial). On the neural level, observed movements that mirror one’s own motor intention should evoke less neural activity during visual processing than observed movements that do not. Indeed, this mirroring effect is likely to attenuate the visual processing of congruent trials compared to incongruent trials. We will refer to these potential processes as the influence of action on perception.
Second, the observed action could affect the participant’s own motor preparation processes, as suggested by many behavioral studies (Brass, Bekkering, et al., 2001; Brass et al., 2000; Stürmer et al., 2000). In other words, observing a movement activates a corresponding motor representation in the observer that can be either compatible with the intended action or incompatible. In the compatible case response selection is facilitated and in the incompatible case it is disturbed. We will refer to these processes as the influence of perception on action (Greenwald, 1970; Hommel et al., 2001; Shin et al., 2010).
Third, assuming that the observed behavior leads to an activation of the corresponding motor representation in the observer, observing a movement that is incongruent to the intended movement can induce conflict that has to be resolved. In other words, it is reasoned that the two motor plans within the cognitive system are conflicting: one that is externally triggered and one that is internally generated. By delineating the internally triggered motor representation from the externally triggered motor representation (Brass, Ruby, & Spengler, 2009), high-level mechanisms might help individuals to distinguish between the self and the observed other. Most imaging studies have focused on this third alternative, namely on resolving conflict between observed and planned movements (Brass et al., 2005; Spengler et al., 2010; Spengler, Von Cramon, et al. 2009a, Spengler, Von Cramon, et al., 2009b). In particular, it has been shown that the temporoparietal junction (TPJ) and medial prefrontal cortex (MPFC) are involved in the imitation inhibition task, brain areas known to engage in self versus other representation (Brass et al., 2005; Sowden & Catmur, 2013; Spengler et al., 2010; Spengler, et al., 2009a, b). This led researchers to relate a social function to these high-level processes dealing with self-other related conflict (Brass et al., 2005, 2009; Santiesteban et al., 2012; Sowden & Catmur, 2013; Spengler et al., 2010; Spengler, et al. 2009a, b).
Yet, functional magnetic resonance imaging (fMRI) might in fact not be sensitive enough to capture subtle effects of action on perception or of perception on action, because of temporal smearing of short-lived effects on a whole-brain level. Electroencephalography (EEG), instead, has a high temporal resolution, which makes it easier to delineate processes on different processing stages. An influence of action on perception should lead to effects in visual event-related potential (ERP) components, whereas an influence of perception on action should impact ERP components related to motor preparation, which appear right before movement execution. Finally, resolving conflict between observed and executed action should lead to congruency effects in central processing stages in the EEG. We concentrated on three functionally distinct ERP components. First, we focused on the stimulus-locked N190, which has been related to visual processing of body parts (Arzy, Thut, Mohr, Michel, & Blanke, 2006; Myers & Sowden, 2008; Thierry et al., 2006). Second, we focused on the response locked Readiness Potential (RP), which is known to magnify with increasing complexities of motor preparation (Leuthold & Schröter, 2011; Rigoni, Brass, Roger, Vidal, & Sartori, 2013). Third, we focused on the central P3 component. In social cognitive paradigms, this component proved sensitive to self-versus-other related processes (Deschrijver, Wiersema, & Brass, 2015; Graux et al., 2013; Knyazev, 2013; Kühn, Nenchev, et al., 2011; Perrin et al., 2005; Sebanz, Knoblich, Prinz, & Wascher, 2006). This makes the component a likely neural correlate of high-level processes of social cognition, which were put forward in fMRI studies (Brass et al., 2005; Santiesteban et al., 2012; Spengler et al., 2010; Spengler, et al. 2009a, b).
Because we specifically wanted to explain the mechanisms that produce the motor interference effect, we also aimed to trace correlations between potential ERP findings and actual task performance (i.e., the congruency effect in the reaction times (RTs) and errors). Moreover, the strength of motor interference effect was often noted as crucial to understanding inadequate control over imitative behaviors in various patient groups (Cook, Barbalat, & Blakemore, 2012; Cook & Bird, 2012; Spengler et al., 2010), including autism spectrum disorder (ASD) (Bird, Leighton, Press, & Heyes, 2007; Cook & Bird, 2012; Cook, Swapp, Pan, Bianchi-Berthouze, & Blakemore, 2014; Gowen, Stanley, & Miall, 2008; Spengler et al., 2010). In the autism domain, it was suggested that individuals with ASD potentially lack high-level social-cognitive self-other distinction, which would lead to increased congruency effects within RTs (hyperimitation effects; Bird et al., 2007; Sowden, Koehne, Catmur, Dziobek, & Bird, 2015; Spengler et al., 2010). However, to date it is has not been tested which neural mechanism contributes to these aberrant motor interference effects in ASD. Therefore, we exploratively assessed the relationship between ERP congruency effects and ASD symptomatology in our non-clinical population, by means of the Autism Quotient (AQ) and Social Responsiveness Scale for adults (SRS-A; Baron-Cohen, Wheelwright, Skinner, Martin, & Clubley, 2001; Bölte, Poustka, & Constantino, 2008). If autistic traits within a neurotypical population are related to high-level self-other distinction (Bird et al., 2007; Sophie Sowden, Koehne, Catmur, Dziobek, & Bird, 2015; Spengler et al., 2010), one would expect correlations between the autism questionnaire scores and the P3 congruency difference.
Method
Participants
A total of 42 healthy volunteers participated in the study. All were right-handed. None had a history of neurological or motor problems. They reported normal or corrected-to-normal vision and normal tactile functioning and hearing. All participants gave written informed consent and were financially compensated for their participation. The local ethics committee approved the study. The data for five participants were excluded because of technical problems during data recording of the EEG signal. The remaining group for EEG analyses consisted of 37 participants (mean age (M) = 22.70 years; standard deviation (SD) = 3.61 years; range = 18−38 years; 13 male). Due to additional technical errors, the behavioral data of four participants could not be included in the behavioral analyses. This left 33 subjects in total for the behavioral analyses.
Design and materials
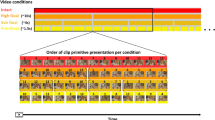
We adopted the established imitation inhibition paradigm used in earlier research (see Fig. 1; Brass et al., 2000, 2001; Spengler, et al. 2009a). Participants were instructed to execute finger movements in response to symbolic cues while observing congruent, incongruent, or no finger movement performed by a left hand positioned on a table (frontal view), presented on the computer screen. In particular, participants had to respond the digit “1” displayed between the index or middle finger of a the hand by lifting their index finger and to a “2” by lifting their middle finger. At the same time the hand on the computer screen executed an index finger movement, a middle finger movement, or no movement at all. In a congruent trial, the participant is required to lift the finger identical to the observed hand’s active finger (e.g., lifting the index finger when an index finger movement is observed). In an incongruent trial, in contrast, the participant is required to lift the finger opposite to the observed hand’s active finger (e.g., lifting the index finger when a middle finger movement is observed). In a baseline trial, the participant is required to lift a finger while the hand does not perform any finger movement.
The study started with a 24-trial practice phase. After this, the experiment started, in which 50 congruent trials (C), 50 incongruent trials (I), and 50 baseline trials (B) were randomly presented. Each trial started with a frame showing a hand in a resting position (2,000 ms), mirroring the right hand of the participant. This frame was followed by two consecutive frames (34 ms each) that showed the finger movement with the number imperative (for congruent and incongruent trials) or just the number imperative (for baseline trials). Then, a picture showing the end position of the hand and the number was shown (1,300 ms). The three movement frames gave the impression of a lifting movement of the index or middle finger, respectively. In between trials, a black screen was presented for 2,000 ms. Intermittent breaks occurred after 50 trials, resulting into two self-paced pauses.
The experiment was conducted in a dimly lit, electrically shielded, and sound-attenuated room. The participant was seated approximately 60 cm from a 17-in. monitor in front of him. The participant’s index and middle fingers of the right hand were placed on the two leftmost finger positions on a response-box with four light sensors. RTs of the onset of the finger-lifting movements were recorded with this device. A keyboard was placed within reach of the left hand. Stimulus delivery and data acquisition were achieved by means of the program Presentation (Neurobs), run on an HP Compaq desktop with Windows XP driver. The data collection for this experiment was part of three different larger studies. In each of the studies, the order of the current experiment was counterbalanced with a second, unrelated experiment.
EEG recording and analyses
The EEG data were recorded with a Biosemi ActiveTwo system (at a sampling rate of 1,024 Hz). We placed 64 active Biosemi EEG electrodes according to the international 10-10 system. Two electrodes were placed on the mastoids for offline rereferencing. To measure eye movements, bipolar electrodes were placed with left and right canthal montage and additionally above and below the left eye. Electrode offsets were kept between −25 and 25 μV at all electrodes. We used BrainVision Analyzer 2 (BVA 2; Brain Products) to analyze the data. After offline re-referencing the data to the average of the left and right mastoid, we applied a high pass filter of 0.1 Hz, a low pass filter of 30 Hz, and a notch filter of 50 Hz. Prior to averaging, the data were automatically corrected for eye movement artifacts by means of the bipolar electrodes around the eyes. An automatic artifact rejection included a gradient check (maximum allowed voltage step: 50 μV/ms within 200 ms before and after the locked event), a minimum/maximum amplitude check (−100 μV and 100 μV, respectively), and a low activity check (0.5 μV within an interval length of 100 ms). Only trials for which the participants produced the correct response between 200 and 1,200 ms after stimulus onset were included in the analyses. We collapsed the data over left- and right-finger movement observations because we were primarily interested in congruency-related processes. We time-locked the stimulus-related ERP components (N190 and P3) to the onset of the first frame with an instruction number (directly following the resting position frame) and the response-related RP to the onset of the participant’s finger movement. All trials received a baseline correction of 100 ms before the respective onset.
All statistical analyses were performed with SPSS Statistics 22. For the N190 and P3, we identified time windows and relevant electrode sites at stable peak topographies (see Fig. 2A) and performed analyses on exported mean area amplitudes. For the N190, we focused on the time window from 170 to 220 ms, and pooled the activity per condition at left hemispheric electrodes P5, P7, and PO7, and at the right hemispheric electrodes P6, P8, and PO8. For the stimulus-locked P3, we pooled the activity at electrodes CPz, Pz, and POz per condition in the time window from 310 to 430 ms. Based on earlier research (Leuthold & Schröter, 2011; Rigoni et al., 2013; Shibasaki & Hallett, 2006), we identified the RP component in the response-locked segments as the gradient shift preceding the steep negative slope before response onset at electrode FCz (i.e., from −400 to −100 ms for the current dataset). To disentangle the activity of the supplementary motor area from motor execution processes in the M1, we increased the spatial resolution of the EEG signal by means of Laplacian transformations (Rigoni et al., 2013; Tandonnet, Burle, Hasbroucq, & Vidal, 2005; Vidal, Grapperon, Bonnet, & Hasbroucq, 2003). We estimated surface Laplacians from the averaged monopolar EEG signal. First, we interpolated the signal with the spherical spline interpolation procedure, and then computed second derivatives in the two dimensions of the space (degree of spline = 3, maximum degrees the Legendre Polynomial = 15). Conforming with earlier studies (e.g., Rigoni et al., 2013; Vidal et al., 2003) and the observed topography (Fig. 2A), we conducted LP-analyses on electrode FCz.
We analyzed results of both behavioral and (pooled) EEG data of the RP and P3 component by means of one-way within-subjects ANOVAs with Condition as a factor (including the levels: B, C, and I). For the N190 EEG data, we additionally included a factor Hemisphere. Greenhouse-Geisser corrections were applied where needed. We used repeated-measures t-tests for paired comparisons. Because of the non-parametric distribution of our effects, Spearman’s correlation coefficients were used for correlational tests. Any differences between congruent/incongruent trials and the baseline trial are to some degree trivial because both the congruent and the incongruent conditions involve movement while the baseline condition does not. Therefore, we decided to mainly focus on the analyses involving the congruent and incongruent conditions.
Results
Behavioral results
As typically described in the imitation inhibition paradigm, we found a significant RT difference for congruency (F(1.33, 42.45) = 60.831, p < 0.001, η2 = 0.66). Participants reacted slower in incongruent trials (M = 503.36 ms; SD = 76.69 ms) than in congruent trials (M = 432.46 ms; SD = 45.55; paired comparisons t(32) = 9.04, p < 0.001), while the RTs of the baseline condition fell in between (M = 471.62 ms; SD = 47.84; respective paired comparisons: t(32) = 11.02, p < 0.001 and t(32) = 4.47, p < 0.001). Analyses on the error percentages (including erroneous as well as missed responses) showed a significant difference for congruency as well (F(1.26; 40.32) = 17.14, p < 0.001, η2 = 0.35). Paired comparisons showed that significantly more errors were made in the incongruent condition (M = 5.47%; SD = 0.06%) than in the congruent condition (M = 0.68%; SD = 0.01%; t(32) = 4.57, p < 0.001) and than in the baseline condition (M = 1.38%; SD = 0.02%; t(32) = 3.99, p < 0.001). No difference between the error rates of the congruent and baseline condition was found (t(32) = 1.44; p = 0.16).
EEG results
N190
The ANOVA with Condition and Hemisphere as factors showed a strong main effect of Condition (F(1.51, 54.24) = 18.28; p < 0.001; partial η2 = 0.34), and of Hemisphere (F(1, 36) = 6.33; p < 0.05; partial η2 = 0.15), yet no interaction of Condition and Hemisphere (F(1.62, 58.39) = 1.15; p = 0.32; partial η2 = 0.03; Fig. 3A, B). The main effect of Hemisphere signified that larger N190 amplitudes were measured at left-lateralized hemisphere sites. Paired t-tests on the conditions collapsed over hemispheres showed that the congruent and incongruent conditions elicited larger amplitudes than the baseline condition (respectively t(36)= 3.99; p < 0.001; and t(36) = 4.99, p < 0.001). Importantly, the t-test on congruent and incongruent trials also yielded a significant result (t(36) = 2.11; p < 0.05), indicating that incongruent trials elicited larger N190 components than congruent trials.
RP
The one-way ANOVA on the RP Laplacians showed a significant difference between the three conditions (F(2, 72) = 5.91, p < 0.005, η2 = 0.14; see Fig. 4A, B). The incongruent condition (M = −16.13 μV/m2, SD = 24.41 μV/m2) elicited larger RP Laplacians than the congruent condition (M = −7.01 μV/m2, SD = 18.42 μV/m2; t(36) = 2.99, p = 0.005). Interestingly, however, the incongruent condition did not differ from the baseline condition (M = −16.46 μV/m2, SD = 3.07 μV/m2; t(36) = −.100, p = .92), whereas the congruent effect did (t(36) = 3.11, p < 0.004). In other words, the observed hand movements yielded response facilitation processes in the congruent condition, but no response interference in the incongruent condition. The congruency effects (I-C) for N190 and RP Laplacian have evidently distinct topographies. The topography maps show that the congruency effect for the N190 (170−220 ms after stimulus onset) is strongest at left parietal sites, while it is most pronounced for the RP Laplacian (300−100 ms before response onset) at fronto-central midline electrodes (see Fig. 2B).
P3
In the P3 component, the ANOVA showed that significant differences existed between the three conditions (F(2,72) = 19.27, p < 0.001, partial η2 = 0.35; see Fig. 5A, B). The congruent trials (pooled average: M = 9.61 μV, SD = 4.80 μV) and the incongruent trials (pooled average: M = 8.57 μV, SD = 4.64 μV) elicited larger P3 amplitudes than baseline trials (pooled average: M = 7.48 μV, SD = 4,47 μV; t(36) = 5.36, p = 0.001 and t(36) = 3.20, p < 0.005, respectively). Incongruent trials elicited smaller P3 amplitudes than congruent trials (t(36) = 3.69, p = 0.001). In other words, observed hand movements that were compatible with own motor intentions yielded larger P3 components than observed hand movements that were incompatible with own motor intentions.
Correlational results
ERP congruency effects and the RT congruency effect
We computed the RT congruency effect for RT (I-C), for the RP (C-I), for the P3 (C-I), and the congruency difference for the N190 pooled over hemispheres (C-I). To avoid detecting correlational effects driven by outliers, we discarded congruency effects from the analyses that were above or below 2.5 SDs from their respective mean, resulting in the exclusion of one participant on the basis of his RT congruency effect and one participant on the basis of his score on the total dimensional scale of the AQ. We then correlated the ERP congruency effects with the RT congruency effect. Interestingly, the P3 effect was positively correlated with the behavioral interference effect (ρ = .45, p < 0.01; see Fig. 6). Individuals with a large congruency effect in the P3 component showed a large behavioral congruency effect. No other correlations reached or trended to significance (both p > 0.16).
ERP congruency effects and non-clinical autistic behaviors
We then correlated the ERP effects with the total dimensional scores on the AQ and on the SRS-A questionnaire. Here, we did not detect any significant correlations (all p > 0.47).
Discussion
Despite almost 15 years of research on the influence of action observation on action execution using interference tasks, the exact mechanisms underlying the motor interference effect are still poorly understood. From a theoretical perspective, three sources might contribute to the interference effect: the influence of action on perception, the influence of perception on action and conflict resolution of the competing representations. While behavioral research has primarily focused on variables that modulate the interference effect (for a review, see Heyes, 2011) or on potential confounds such as spatial compatibility (e.g., Brass et al., 2000), not much research has directly addressed the specific sources of the effect. By contrast, fMRI research has primarily focused on one potential source of the interference effect, namely on conflict resolution between the planned and observed action (Brass et al., 2005, Spengler, et al. 2009a, b; Spengler et al., 2010).
In the current study we used EEG to delineate three potential sources of motor interference. We argue that EEG is more sensitive to subtle differences on the perceptual and motor level, because it allows differentiating these processes in the temporal domain.
The imitation inhibition paradigm has been investigated only once using EEG. This study, however, focused on emotion perception, rather than on the mechanisms of automatic imitation (Grecucci, Balaban, Buiatti, Budai, & Rumiati, 2009). The current study assessed the original imitation inhibition paradigm (Brass et al., 2000; Brass & Heyes, 2005) by means of EEG. We focused on three EEG components that should in our opinion index the three potential sources of the interference effect, namely the visual N190 indexing the influence of action on perception, the motor-related RP indexing the influence of perception on action, and the P3 component indexing conflict resolution. Interestingly, we detected congruency effects in all of these ERP components, suggesting that all aforementioned processes play a role in the imitation inhibition task. To our knowledge this is the first evidence showing that different sources contribute to interference effects in the imitation inhibition task.
The effect of action on perception
First, we aimed to detect the effect of action on perception within the amplitudes of the N190, which had been related to activity in the extrastriate body area (EBA) of the visual system (Thierry et al., 2006). Over hemispheres, N190 amplitudes were larger for congruent and incongruent trials, in which an imperative stimulus and a finger movement were displayed, compared to baseline trials, where an imperative stimulus but no finger movement was displayed. Evaluating the difference between the congruent and incongruent condition and the baseline condition is not very informative here because it compares two conditions that show movement with a condition that does not show movement.
Importantly, the N190 components showed larger amplitudes for incongruent than for congruent trials. In other words, hand actions that were compatible to one’s own action intention evoked less brain activation related to the visual processing of body-parts than hand actions incompatible to the action intention. This suggests that compatible observed hand actions required less visual processing “effort” than the incompatible ones, leading to larger N190 amplitudes for the latter. In other words, a compatible action intention might have facilitated the visual processing of observed congruent finger movements. As an alternative interpretation, one could assume that the N190 effect could reflect processes of visual (Vocks et al., 2010) or embodied self-other discrimination, which is considered as functionally distinct from high-level, more cognitive self-other distinction (Arzy et al., 2006). Indeed, it has earlier been described that the EBA, which typically underlies the N190 amplitudes (Thierry et al., 2006), responds more strongly to movements that are clearly someone else’s (David et al., 2009; Myers & Sowden, 2008; Stanley & Miall, 2007; but see Vocks et al., 2010). Drawing conclusions from the baseline condition of the imitation inhibition task (see earlier), we can for now not disentangle these two potential interpretations. It is noteworthy though that an interpretation in terms of visual self-other distinction does not necessarily go against an interpretation in terms of action effects on perception. Indeed, when the expected visual consequences of one’s action intentions facilitate the actual visual observation of a hand moving (congruent trials), the observed hand action is more likely to be part of one’s own body. Similarly, when the expected visual consequences of one’s action intention do not match the observed hand movement, the visual processing thereof becomes more effortful and the observed hand is not likely to be one’s own.
This finding is consistent with recent studies which showed that the compatibility of cued action intentions modulates the visual processing of subsequently observed actions, as reflected in an ERP-component similar to the N190 (Bortoletto, Mattingley, & Cunnington, 2011; Press, Gherri, Heyes, & Eimer, 2010) and in brain activity in V1 as measured in an fMRI study (Stanley & Miall, 2007). In aforementioned EEG studies that reported an interaction of action observation and action intentions within early visual components (Bortoletto et al., 2011; Press et al., 2010), participants were told which response to prepare before the visual action stimulus was presented. In contrast to these studies, participants in our study did not know which response to prepare before the imperative stimulus was presented. An effect of action on perception by 190 ms in our study can therefore be considered as very quick: It suggests that the imperative information has reached motor preparation areas and has also fed back to perceptual processes within this short period of time. It should however be noted that the reaction times reflect both response selection and response execution processes. It is likely that the effect of action on perception is affected by early response selection processes rather than response execution processes (see also below).
This finding also suggests that the N190 component responded to trials showing motion of hands and not just to trials showing hands per se. Because the neutral hand posture had been displayed from the start of the trial, the neural activity within the N190 component for congruent and incongruent conditions can only be due to the observed movement of the hand. Given the limited spatial resolution of EEG, this result may not be surprising: the MT+ area in the human, which is dedicated to the visual processing of biological actions, is known to show some overlap with the EBA (Ferri, Kolster, Jastorff, & Orban, 2013). It is therefore reasonable to assume that the N190 component might have picked up activity coming from this latter area as well. Similar findings were reported in other studies, with action observations (Bortoletto et al., 2011; Press et al., 2010) and changes in body configuration (Borhani, Borgomaneri, Làdavas, & Bertini, 2016; Borhani, Làdavas, Maier, Avenanti, & Bertini, 2015) leading to larger N190 components. Additionally, it could be noted that an increased visual saliency of the congruency conditions compared to the baseline condition (which did not contain visual movement) might account for this difference. Given the known association between activity within the N190 and biological (body-related) processes, we consider the possibility that the N190 modulation reflected the visual processing of “mere” (non-biological) movement unlikely (though we cannot fully discard it based on our design alone; see also Press et al., 2010).
Overall, the current N190 results add to findings which showed that action representations of own movements influence different stages of perception (Calvo-Merino, Grèzes, Glaser, Passingham, & Haggard, 2006; Craighero, Fadiga, Rizzolatti, & Umiltà, 1999; Hamilton, Wolpert, & Frith, 2004; Kühn, Keizer, Rombouts, & Hommel, 2011a; Schütz-Bosbach & Prinz, 2007; Thomaschke, 2012). Interestingly, fMRI studies on the imitation-inhibition task so far did not reveal activation in EBA/MT (Brass et al., 2005; Kontaris, Wiggett, & Downing, 2009; Spengler et al., 2010; Spengler, et al. 2009a, b). We think that this is due to the fact that fMRI is less sensitive to such subtle changes.
The effect of perception on action
Next, we focused on low-level mechanisms of imitative control at the level of action preparation, as reflected in the RP. Confirming our hypothesis, we detected a congruency effect within the Laplacian RP (Leuthold & Schröter, 2011; Rigoni et al., 2013; Shibasaki & Hallett, 2006). The congruent trials elicited smaller RP Laplacians than the incongruent trials and baseline trials. We did not detect a significant difference between the incongruent and the baseline conditions. This suggests that the observation of incompatible movements did not disturb response selection processes, as compared to observing no movement at all. The results may thus show that the preparation of own actions was facilitated when the observed hand movement matched the intended one. As such, the data would reveal a facilitation mechanism for congruent trials at the level of motor selection. However, also here, one should be careful with drawing conclusions from the baseline condition. Overall, with the current results we confirm that action perception influences the preparation of own movements, as was predicted by various theoretical works (Brass & Heyes, 2005; Greenwald, 1970; Hommel et al., 2001; Rizzolatti & Craighero, 2004; Shin et al., 2010).
High-level cognitive processes: P3 results
Finally, we observed a congruency effect in the P3 component, which we put forward as a likely neural correlate for self-other distinction processes. In the current study, we showed that congruent trials elicited larger P3 amplitudes than incongruent trials. As we assume that self-other distinction is required to distinguish the intended from the externally triggered motor plan, the congruency difference may reflect the conflict between the two motor plans. The findings suggest that the P3 was most sensitive to the condition in which the observed action was consistent with the intended action of the participant. The baseline condition, which did not present any hand movement, elicited the smallest P3 amplitude, potentially suggesting that the brain might have perceived it as least compatible to one’s own action intention or that this condition was less visually salient (conforming with findings in oddball tasks, e.g., see Donchin, 1981).
The current results follow earlier EEG findings in social cognitive paradigms which reported larger P3 amplitudes for congruent trials in the context of self-versus-other processing (e.g., Holeckova et al., 2006; Longo et al., 2012) such as hearing one’s own name or seeing one’s own face (Cygan, Tacikowski, Ostaszewski, Chojnicka, & Nowicka, 2014; Holeckova, Fischer, Giard, Delpuech, & Morlet, 2006; Perrin et al., 2005; Tacikowski, Cygan, & Nowicka, 2014; Tacikowski, Jednoróg, Marchewka, & Nowicka, 2011; Tacikowski & Nowicka, 2010), or perceiving touch/seeing actions that are compatible with one’s own touch/own actions (de la Asuncion, Bervoets, Morrens, Sabbe, & De Bruijn, 2015; Deschrijver et al., 2015; Longo, Musil, & Haggard, 2012; Ruissen & de Bruijn, 2015). Studies that have focused on self-other related conflict in the somatosensory domain reported similar modulations in brain activity around 300 ms at parietal sites (Longo et al., 2012; Papeo, Longo, Feurra, & Haggard, 2010). It is noteworthy that in the non-social domain it is a common observation that the parietal P3 is smaller for incongruent versus congruent trials (e.g., Hillman, Belopolsky, Snook, Kramer, & McAuley, 2004; Hillman, Snook, & Jerome, 2003; Mahé, Doignon-Camus, Dufour, & Bonnefond, 2014; Neuhaus et al., 2010) even though surprising events generally elicit larger P3 components (Donchin, 1981). These findings have been explained in terms of an increased need for interference control in the incongruent condition, leading to less available resources that are also needed for generating the P3 component (Kok, 2001; Polich, 2007). Similarly, one could hypothesize that a mechanism resolving the conflict between own actions and incompatible observed actions would lead to smaller P3 components in the context of self-versus-other related high-level processes.
Our findings are also consistent with previous fMRI studies implicating the role of self-other distinction in the imitation inhibition paradigm (Brass et al., 2009; Santiesteban et al., 2012; Sowden & Catmur, 2013; Spengler et al., 2010; Spengler, et al. 2009a). Moreover, though the sources of the P3-component are difficult to localize, the temporo-parietal junction (TPJ), the medial prefrontal cortex (mPFC), and the precuneus have been named as potential neural underpinnings (Knyazev, 2013; Mulert et al., 2004; Papeo et al., 2010; Perrin et al., 2005; Verleger, Jaśkowski, & Wascher, 2005). These brain areas were deemed important in self-other distinction processes by the aforementioned fMRI studies of the imitation inhibition paradigm (Brass et al., 2005; Spengler, et al. 2009a).
In sum, the P3 findings contribute vastly to earlier fMRI studies of the imitation inhibition task, by not only confirming the involvement of high-level conflict-related processes in the task but also by clarifying the timing thereof (Brass et al., 2009; Santiesteban et al., 2012; S. Sowden & Catmur, 2013; Spengler et al., 2010; Spengler, et al. 2009a, b).
Relative timing of the neural processes
As can be seen in Fig. 4A, the RP has an early onset around 350 ms before the participant’s response. While the RP is locked to this response and mean reaction times of congruent and incongruent processes are around 430 ms and 500 ms, respectively, the onset of the early readiness potential is about 80−150 ms after stimulus presentation. The N190 peaks about 190 ms post stimulus. As such, it can be speculated that early response selection processes starting around 80−150 ms after stimulus onset feed back to the visual processing of the observed hand action (Borhani et al., 2015; Bortoletto et al., 2011; Press et al., 2010). In other words, we assume that early motor planning processes (response selection) influence perception of biological motion, which is reflected in the congruency effect of the N190. Congruency effects in the RP are observed a little bit later: Visual processing of the congruent and incongruent movement might therefore influence later stages of motor preparation, as reflected in the congruency effect within RP. Finally, the P3 effect is largest around 300−400 ms. We think the conflict between the intended and externally triggered motor representations drives the P3. In other words, the P3 may reflect the high-level social cognitive processes which delineate the external motor representation from the internally generated one (Brass et al., 2009; Brass, Zysset, et al., 2001; Spengler et al., 2010). The timing of the P3 is consistent with the functional interpretations thereof which assume that it is influenced by perceptual, motor, and stimulus decision processes (Verleger et al., 2005).
Correlational results
As a final goal of this study, we wanted to investigate which of the three mechanisms identified above contributed most to the behavioral motor interference effect and which of these mechanisms could be related to autistic traits. By means of correlation analyses we provided support for a functional link between the motor interference effect and the P3 effect. This suggests that the more individuals were able to distinguish between congruent and incongruent trials at high levels of processing, the more interference they experienced on a behavioral level.
Contrary to expectations, we did not detect significant correlational findings between the ERP congruency effects and social autistic traits (Brass et al., 2009; Spengler et al., 2010; Spengler, et al. 2009a). As such, we would not confirm the hypothesis that high-level social-cognitive self-other distinction is associated with (non-clinical) autistic traits. Though one should be careful with interpreting a null result in this non-clinical population, it might suggest that behavioral hyperimitation found for groups with higher autistic traits (Bird et al., 2007; Sowden et al., 2015; Spengler et al., 2010) may be related to neural processes other than high-level social-cognitive ones.
Limitations
It is worth noting that all three components have some temporal overlap. However, they can be distinguished on the basis of their topography and temporal signature. In the literature, the cognitive processes that are assumed to drive the effects in the N190, P3, and RP components are considered to be functionally distinct: The N190 has strongly been related to the processing of body-related visual information (Thierry et al., 2006) and has been related to the EBA/MT complex in the temporal cortex (Borhani et al., 2016, 2015; Thierry et al., 2006). The P3 component has been related to stimulus evaluation, decision, novelty processing, and working memory updating (Friedman, Cycowicz, & Gaeta, 2001; Polich, 2007; Verleger et al., 2005), and to processes linking perception to action (Verleger et al., 2005). Frontoparietal areas including TPJ (Mulert et al., 2004; Volpe et al., 2007) have been related to the P3. The RP on the other hand has thoroughly been discussed in terms of motor preparation processes (e.g., Leuthold & Schröter, 2011; Shibasaki & Hallett, 2006). It is assumed that the (pre-)SMA is related to the early readiness potential and the premotor cortex and primary motor cortex are related to the late readiness potential (Leuthold & Schröter, 2011; Rigoni et al., 2013; Schröter & Leuthold, 2009; Shibasaki & Hallett, 2006; Xu, Sommer, & Masaki, 2015). Therefore, we consider it unlikely that the reported effects are not functionally dissociated, an interpretation that is also supported by the distinct topographical maps for the N190 and Laplacian RP effects (see Fig. 2B).
In addition, it is theoretically possible that processes related to the imperative cue that are not linked to motor preparation cause our effects. In this case, our results would be generated by non-motor cognitive processes (i.e., linguistic or symbolic ones). However, we do not think that this is very likely. First, there is no semantic overlap between the imperative stimuli (i.e., numbers) and the finger movements. In this respect the effect is very different from a Stroop effect, for example. Second, RTs in this task are very fast, making it very unlikely that the symbolic cue is first translated in a semantic representation which then triggers the motor program. Because we have no direct experimental evidence for these claims, we nevertheless chose to present the alternative interpretation here.
Conclusion
The current EEG study expands findings of fMRI studies focusing on the imitation inhibition task (Brass et al., 2005; Spengler et al., 2010; Spengler, et al. 2009a) by identifying a role of low-level visual and motor preparation processes in the imitation inhibition task. As such, it underscores various theories that assume a strong linkage between low-level visual processes and low-level action preparation (Brass et al., 2009; Hommel et al., 2001; Shin et al., 2010). Additionally, our correlational findings suggest that the P3 is functionally related to the RT congruency effect. We could, however, not identify a relationship between the ERP congruency effects and non-clinical autistic traits (Bird et al., 2007; Cook & Bird, 2012; Cook et al., 2014; Gowen et al., 2008; Spengler et al., 2010). Overall, our study implies the existence of functionally distinct effects of perception on action, action on perception, and high-level self-other distinction within the imitation inhibition task.
References
Arzy, S., Thut, G., Mohr, C., Michel, C. M., & Blanke, O. (2006). Neural basis of embodiment: Distinct contributions of temporoparietal junction and extrastriate body area. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 26(31), 8074–8081. doi:10.1523/JNEUROSCI.0745-06.2006
Baron-Cohen, S., Wheelwright, S., Skinner, R., Martin, J., & Clubley, E. (2001). The Autism-Spectrum Quotient (AQ): Evidence from Asperger Syndrome/High-Functioning Autism, Males and Females, Scientists and Mathematicians. Journal of Autism and Developmental Disorders, 31(1), 5–17. doi:10.1023/A:1005653411471
Bertenthal, B. I., Longo, M. R., & Kosobud, A. (2006). Imitative response tendencies following observation of intransitive actions. Journal of Experimental Psychology: Human Perception and Performance, 32(2), 210–225. doi:10.1037/0096-1523.32.2.210
Bird, G., Leighton, J., Press, C., & Heyes, C. (2007). Intact automatic imitation of human and robot actions in autism spectrum disorders. Proceedings of the Royal Society B: Biological Sciences, 274, 3027–3031. doi:10.1098/rspb.2007.1019
Bölte, S., Poustka, F., & Constantino, J. N. (2008). Assessing autistic traits: Cross-cultural validation of the social responsiveness scale (SRS). Autism Research, 1, 354–363. doi:10.1002/aur.49
Borhani, K., Borgomaneri, S., Làdavas, E., & Bertini, C. (2016). The effect of alexithymia on early visual processing of emotional body postures. Biological Psychology, 115, 1–8. doi:10.1016/j.biopsycho.2015.12.010
Borhani, K., Làdavas, E., Maier, M. E., Avenanti, A., & Bertini, C. (2015). Emotional and movement-related body postures modulate visual processing. Social Cognitive and Affective Neuroscience, 10(8), 1092–1101. doi:10.1093/scan/nsu167
Bortoletto, M., Mattingley, J. B., & Cunnington, R. (2011). Action intentions modulate visual processing during action perception. Neuropsychologia, 49(7), 2097–2104. doi:10.1016/j.neuropsychologia.2011.04.004
Brass, M., Bekkering, H., Wohlschläger, A., & Prinz, W. (2000). Compatibility between observed and executed finger movements: Comparing symbolic, spatial, and imitative cues. Brain and Cognition, 44, 124–143. doi:10.1006/brcg.2000.1225
Brass, M., Bekkering, H., & Prinz, W. (2001). Movement observation affects movement execution in a simple response task. Acta Psychologica, 106, 3–22. doi:10.1016/S0001-6918(00)00024-X
Brass, M., Derrfuss, J., Matthes-von Cramon, G., & von Cramon, D. Y. (2003). Imitative response tendencies in patients with frontal brain lesions. Neuropsychology, 17(2), 265–271.
Brass, M., Derrfuss, J., & Von Cramon, D. Y. (2005). The inhibition of imitative and overlearned responses: A functional double dissociation. Neuropsychologia, 43, 89–98. doi:10.1016/j.neuropsychologia.2004.06.018
Brass, M., & Heyes, C. (2005). Imitation: Is cognitive neuroscience solving the correspondence problem? Trends in Cognitive Sciences, 9(10), 489–495. doi:10.1016/j.tics.2005.08.007
Brass, M., Ruby, P., & Spengler, S. (2009). Inhibition of imitative behaviour and social cognition. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 364, 2359–2367. doi:10.1098/rstb.2009.0066
Brass, M., Zysset, S., & von Cramon, D. Y. (2001). The inhibition of imitative response tendencies. NeuroImage, 14, 1416–1423. doi:10.1006/nimg.2001.0944
Calvo-Merino, B., Grèzes, J., Glaser, D. E., Passingham, R. E., & Haggard, P. (2006). Seeing or doing? Influence of visual and motor familiarity in action observation. Current Biology, 16, 1905–1910. doi:10.1016/j.cub.2006.07.065
Cook, J. L., Barbalat, G., & Blakemore, S.-J. (2012). Top-down modulation of the perception of other people in schizophrenia and autism. Frontiers in Human Neuroscience, 6, 1–10. doi:10.3389/fnhum.2012.00175
Cook, J. L., & Bird, G. (2012). Atypical social modulation of imitation in autism spectrum conditions. Journal of Autism and Developmental Disorders, 42, 1045–1051. doi:10.1007/s10803-011-1341-7
Cook, J., Swapp, D., Pan, X., Bianchi-Berthouze, N., & Blakemore, S.-J. (2014). Atypical interference effect of action observation in autism spectrum conditions. Psychological Medicine, 44, 731–740. doi:10.1017/S0033291713001335
Craighero, L., Fadiga, L., Rizzolatti, G., & Umiltà, C. (1999). Action for perception: A motor-visual attentional effect. Journal of Experimental Psychology, Human Perception and Performance, 25(6), 1673–1692.
Cygan, H. B., Tacikowski, P., Ostaszewski, P., Chojnicka, I., & Nowicka, A. (2014). Neural correlates of own name and own face detection in autism spectrum disorder. PLoS ONE, 9(1). doi:10.1371/journal.pone.0086020
David, N., Jansen, M., Cohen, M. X., Osswald, K., Molnar-Szakacs, I., Newen, A., … Paus, T. (2009). Disturbances of self-other distinction after stimulation of the extrastriate body area in the human brain. Social Neuroscience, 4, 40–48. doi:10.1080/17470910801938023
de la Asuncion, J., Bervoets, C., Morrens, M., Sabbe, B., & De Bruijn, E. R. A. (2015). EEG correlates of impaired self-other integration during joint-task performance in schizophrenia. Social Cognitive and Affective Neuroscience, 10(10), 1365–1372. doi:10.1093/scan/nsv023
Deschrijver, E., Wiersema, J. R., & Brass, M. (2015). The interaction between felt touch and tactile consequences of observed actions: An action-based somatosensory congruency paradigm. Social Cognitive and Affective Neuroscience. doi:10.1093/scan/nsv081
Donchin, E. (1981). Surprise!… Surprise? Psychophysiology. doi:10.1111/j.1469-8986.1981.tb01815.x
Ferri, S., Kolster, H., Jastorff, J., & Orban, G. (2013). The overlap of the EBA and the MT/V5 cluster. NeuroImage, 66, 412–425. doi:10.1016/j.neuroimage.2012.10.060
Friedman, D., Cycowicz, Y. M., & Gaeta, H. (2001). The novelty P3: An event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neuroscience and Biobehavioral Reviews, 25, 355–373. doi:10.1016/S0149-7634(01)00019-7
Gowen, E., Stanley, J., & Miall, R. C. (2008). Movement interference in autism-spectrum disorder. Neuropsychologia, 46, 1060–1068. doi:10.1016/j.neuropsychologia.2007.11.004
Graux, J., Gomot, M., Roux, S., Bonnet-Brilhault, F., Camus, V., & Bruneau, N. (2013). My voice or yours? An electrophysiological study. Brain Topography, 26, 72–82. doi:10.1007/s10548-012-0233-2
Grecucci, A., Balaban, E., Buiatti, T., Budai, R., & Rumiati, R. I. (2009). The emotional control of action: ERP evidence. Archives Italiennes de Biologie, 147, 37–49.
Greenwald, A. G. (1970). Sensory feedback mechanisms In performance control: With special reference to the ideo-motor mechanism. Psychological Review, 77(2), 73–99.
Hamilton, A., Wolpert, D., & Frith, U. (2004). Your own action influences how you perceive another person’s action. Current Biology, 14, 493–498. doi:10.1016/j
Heyes, C. (2011). Automatic imitation. Psychological Bulletin, 137(3), 463–483. doi:10.1037/a0022288
Heyes, C., Bird, G., Johnson, H., & Haggard, P. (2005). Experience modulates automatic imitation. Cognitive Brain Research, 22(2), 233–240. doi:10.1016/j.cogbrainres.2004.09.009
Hillman, C. H., Belopolsky, A. V., Snook, E. M., Kramer, A. F., & McAuley, E. (2004). Physical activity and executive control: Implications for increased cognitive health during older adulthood. Research Quarterly for Exercise and Sport, 75(2), 176–185. doi:10.1080/02701367.2004.10609149
Hillman, C. H., Snook, E. M., & Jerome, G. J. (2003). Acute cardiovascular exercise and executive control function. International Journal of Psychophysiology, 48(3), 307–314. doi:10.1016/S0167-8760(03)00080-1
Holeckova, I., Fischer, C., Giard, M. H., Delpuech, C., & Morlet, D. (2006). Brain responses to a subject’s own name uttered by a familiar voice. Brain Research, 1082, 142–152. doi:10.1016/j.brainres.2006.01.089
Hommel, B., Müsseler, J., Aschersleben, G., & Prinz, W. (2001). The Theory of Event Coding (TEC): A framework for perception and action planning. The Behavioral and Brain Sciences, 24, 849–878. doi:10.1017/S0140525X01000103. discussion 878–937.
Knyazev, G. G. (2013). EEG correlates of self-referential processing. Frontiers in Human Neuroscience, 7, 1–14. doi:10.3389/fnhum.2013.00264
Kok, A. (2001). On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology, 38(03), 557–577. doi:10.1016/S0167-8760(98)90168-4
Kontaris, I., Wiggett, A. J., & Downing, P. E. (2009). Dissociation of extrastriate body and biological-motion selective areas by manipulation of visual-motor congruency. Neuropsychologia, 47(14), 3118–3124. doi:10.1016/j.neuropsychologia.2009.07.012
Kühn, S., Keizer, A., Rombouts, S. A. R. B., & Hommel, B. (2011). The functional and neural mechanism of action preparation: Roles of EBA and FFA in voluntary action control. Journal of Cognitive Neuroscience, 23(1), 214–220. doi:10.1162/jocn.2010.21418
Kühn, S., Nenchev, I., Haggard, P., Brass, M., Gallinat, J., & Voss, M. (2011). Whodunnit? Electrophysiological correlates of agency judgements. PLoS ONE, 6(12), 1–6. doi:10.1371/journal.pone.0028657
Leuthold, H., & Schröter, H. (2011). Motor programming of finger sequences of different complexity. Biological Psychology, 86(1), 57–64. doi:10.1016/j.biopsycho.2010.10.007
Longo, M., Musil, J., & Haggard, P. (2012). Visuo-tactile integration in personal space. Journal of Cognitive Neuroscience, 24(3), 543–552. doi:10.1162/jocn_a_00158
Mahé, G., Doignon-Camus, N., Dufour, A., & Bonnefond, A. (2014). Conflict control processing in adults with developmental dyslexia: An event related potentials study. Clinical Neurophysiology, 125(1), 69–76. doi:10.1016/j.clinph.2013.06.005
Mulert, C., Pogarell, O., Juckel, G., Rujescu, D., Giegling, I., Rupp, D., … Hegerl, U. (2004). The neural basis of the P300 potential: Focus on the time-course of the underlying cortical generators. European Archives of Psychiatry and Clinical Neuroscience, 254, 190–198. doi:10.1007/s00406-004-0469-2
Myers, A., & Sowden, P. T. (2008). Your hand or mine? The extrastriate body area. NeuroImage, 42, 1669–1677. doi:10.1016/j.neuroimage.2008.05.045
Neuhaus, A. H., Urbanek, C., Opgen-Rhein, C., Hahn, E., Ta, T. M. T., Koehler, S., … Dettling, M. (2010). Event-related potentials associated with Attention Network Test. International Journal of Psychophysiology, 76(2), 72–79. doi:10.1016/j.ijpsycho.2010.02.005
Papeo, L., Longo, M. R., Feurra, M., & Haggard, P. (2010). The role of the right temporoparietal junction in intersensory conflict: Detection or resolution? Experimental Brain Research, 206, 129–139. doi:10.1007/s00221-010-2198-2
Perrin, F., Maquet, P., Peigneux, P., Ruby, P., Degueldre, C., Balteau, E., … Laureys, S. (2005). Neural mechanisms involved in the detection of our first name: A combined ERPs and PET study. Neuropsychologia, 43, 12–19. doi:10.1016/j.neuropsychologia.2004.07.002
Polich, J. (2007). Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 118(10), 2128–2148. doi:10.1016/j.clinph.2007.04.019
Press, C., Gherri, E., Heyes, C., & Eimer, M. (2010). Action preparation helps and hinders perception of action. Journal of Cognitive Neuroscience, 22(10), 2198–2211.
Rigoni, D., Brass, M., Roger, C., Vidal, F., & Sartori, G. (2013). Top-down modulation of brain activity underlying intentional action and its relationship with awareness of intention: An ERP/Laplacian analysis. Experimental Brain Research, 229, 347–357. doi:10.1007/s00221-013-3400-0
Rizzolatti, G., & Craighero, L. (2004). The mirror-neuron system. Annual Review of Neuroscience, 27, 169–192. doi:10.1146/annurev.neuro.27.070203.144230
Ruissen, M. I., & de Bruijn, E. R. A. (2015). Is it me or is it you? Behavioral and electrophysiological effects of oxytocin administration on self-other integration during joint task performance. Cortex; A Journal Devoted to the Study of the Nervous System and Behavior, 70, 146–154. doi:10.1016/j.cortex.2015.04.017
Santiesteban, I., White, S., Cook, J., Gilbert, S. J., Heyes, C., & Bird, G. (2012). Training social cognition: From imitation to Theory of Mind. Cognition, 122(2011), 228–235. doi:10.1016/j.cognition.2011.11.004
Schröter, H., & Leuthold, H. (2009). Motor programming of rapid finger sequences: Inferences from movement-related brain potentials. Psychophysiology, 46, 388–401. doi:10.1111/j.1469-8986.2008.00772.x
Schütz-Bosbach, S., & Prinz, W. (2007). Perceptual resonance: Action-induced modulation of perception. Trends in Cognitive Sciences, 11(8), 349–355. doi:10.1016/j.tics.2007.06.005
Sebanz, N., Knoblich, G., Prinz, W., & Wascher, E. (2006). Twin peaks: An ERP study of action planning and control in co-acting individuals. Journal of Cognitive Neuroscience, 18, 859–870. doi:10.1162/jocn.2006.18.5.859
Shibasaki, H., & Hallett, M. (2006). What is the Bereitschaftspotential? Clinical Neurophysiology, 117, 2341–2356. doi:10.1016/j.clinph.2006.04.025
Shin, Y. K., Proctor, R. W., & Capaldi, E. J. (2010). A review of contemporary ideomotor theory. Psychological Bulletin, 136(6), 943–974. doi:10.1037/a0020541
Sowden, S., & Catmur, C. (2013). The role of the right temporoparietal junction in the control of imitation. Cerebral Cortex, 2010, 1–7. doi:10.1093/cercor/bht306
Sowden, S., Koehne, S., Catmur, C., Dziobek, I., & Bird, G. (2015). Intact automatic imitation and typical spatial compatibility in autism spectrum disorder: Challenging the broken mirror theory. Autism Research. doi:10.1002/aur.1511
Spengler, S., Bird, G., & Brass, M. (2010). Hyperimitation of actions is related to reduced understanding of others’ minds in autism spectrum conditions. Biological Psychiatry, 68, 1148–1155. doi:10.1016/j.biopsych.2010.09.017
Spengler, S., Von Cramon, D. Y., & Brass, M. (2009a). Control of shared representations relies on key processes involved in mental state attribution. Human Brain Mapping, 30(June), 3704–3718. doi:10.1002/hbm.20800
Spengler, S., von Cramon, D. Y., & Brass, M. (2009b). Was it me or was it you? How the sense of agency originates from ideomotor learning revealed by fMRI. NeuroImage, 46(1), 290–298. doi:10.1016/j.neuroimage.2009.01.047
Stanley, J., & Miall, R. C. (2007). Functional activation in parieto-premotor and visual areas dependent on congruency between hand movement and visual stimuli during motor-visual priming. NeuroImage, 34(1), 290–299. doi:10.1016/j.neuroimage.2006.08.043
Stürmer, B., Aschersleben, G., & Prinz, W. (2000). Correspondence effects with manual gestures and postures: A study of imitation. Journal of Experimental Psychology. Human Perception and Performance, 26(6), 1746–1759.
Tacikowski, P., Cygan, H. B., & Nowicka, A. (2014). Neural correlates of own and close-other’s name recognition: ERP evidence. Frontiers in Human Neuroscience, 8(April), 1–10. doi:10.3389/fnhum.2014.00194
Tacikowski, P., Jednoróg, K., Marchewka, A., & Nowicka, A. (2011). How multiple repetitions influence the processing of self-, famous and unknown names and faces: An ERP study. International Journal of Psychophysiology, 79(2), 219–230. doi:10.1016/j.ijpsycho.2010.10.010
Tacikowski, P., & Nowicka, A. (2010). Allocation of attention to self-name and self-face: An ERP study. Biological Psychology, 84(2), 318–324. doi:10.1016/j.biopsycho.2010.03.009
Tandonnet, C., Burle, B., Hasbroucq, T., & Vidal, F. (2005). Spatial enhancement of EEG traces by surface Laplacian estimation: Comparison between local and global methods. Clinical Neurophysiology, 116, 18–24. doi:10.1016/j.clinph.2004.07.021
Thierry, G., Pegna, A. J., Dodds, C., Roberts, M., Basan, S., & Downing, P. (2006). An event-related potential component sensitive to images of the human body. NeuroImage, 32, 871–879. doi:10.1016/j.neuroimage.2006.03.060
Thomaschke, R. (2012). Investigating ideomotor cognition with motorvisual priming paradigms: Key findings, methodological challenges, and future directions. Frontiers in Psychology, 3, 1–15. doi:10.3389/fpsyg.2012.00519
Verleger, R., Jaśkowski, P., & Wascher, E. (2005). Evidence for an integrative role of P3b in linking reaction to perception. Journal of Psychophysiology, 19(3), 165–181. doi:10.1027/0269-8803.19.3.165
Vidal, F., Grapperon, J., Bonnet, M., & Hasbroucq, T. (2003). The nature of unilateral motor commands in between-hand choice tasks as revealed by surface Laplacian estimation. Psychophysiology, 40, 796–805. doi:10.1111/1469-8986.00080
Vocks, S., Busch, M., Grönemeyer, D., Schulte, D., Herpertz, S., & Suchan, B. (2010). Differential neuronal responses to the self and others in the extrastriate body area and the fusiform body area. Cognitive, Affective & Behavioral Neuroscience, 10(3), 422–429. doi:10.3758/CABN.10.3.422
Volpe, U., Mucci, A., Bucci, P., Merlotti, E., Galderisi, S., & Maj, M. (2007). The cortical generators of P3a and P3b: A LORETA study. Brain Research Bulletin, 73, 220–230. doi:10.1016/j.brainresbull.2007.03.003
Xu, L., Sommer, W., & Masaki, H. (2015). The structure of motor programming: Evidence from reaction times and lateralized readiness potentials. Psychophysiology, 52, 149–155. doi:10.1111/psyp.12296
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deschrijver, E., Wiersema, J.R. & Brass, M. The influence of action observation on action execution: Dissociating the contribution of action on perception, perception on action, and resolving conflict. Cogn Affect Behav Neurosci 17, 381–393 (2017). https://doi.org/10.3758/s13415-016-0485-5
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-016-0485-5